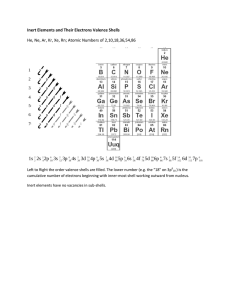
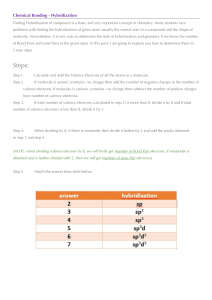
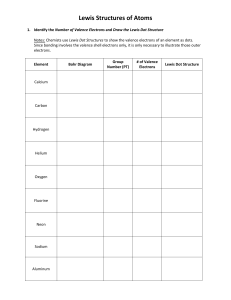
Naroe I 'i'/hot 2, I-low Evaluating does expec+ to electror,E group 3. same amount of orbitals/rings of electrons period of Bohr Diagrams ond Electron Dot Diagrams fifth period? fif'*.cl 5 in same number of valence electrons Mende'eev orgr„nized Krowr.g Thot The ord Pe-iodic Table on eiecTrone deterrn ne properties OF elements, bonds To does This sense? valence electrons determine chemical properties 5 Lli+irna+e gain cr have 8 t'nat eiectrof'i*, r eiernenfs c,bi'.i+h' from group I To are often bonded tc elements from. so they can gain an electron eroup 170 The is idea why doefi group 18 unreact've? thier valence shells are "filled" and they are the inert gases