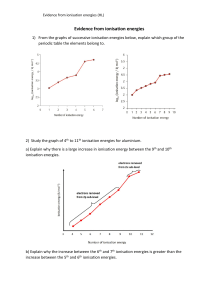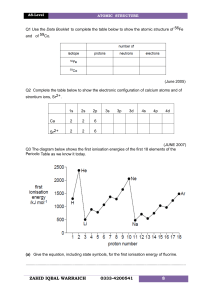
IONISATION ENERGY – HIGHER HIGHER Circle the element with the higher first ionisation energy and explain why it is higher. Include the electron structure of the atom/ion losing an electron. circle the higher one 1 argon v potassium 2 phosphorus v sulfur 3 magnesium v calcium 4 magnesium v aluminium 5 oxygen v fluorine explanation Circle the element with the higher second ionisation energy and explain why it is higher. Include the electron structure of the atom/ion losing an electron. circle the higher one 6 sodium v magnesium 7 neon v sodium 8 phosphorus v sulfur © www.CHEMSHEETS.co.uk explanation 23-September-2018 Chemsheets AS 1219