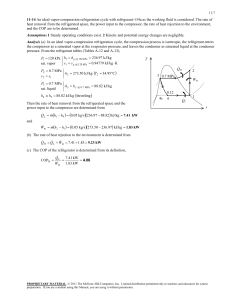
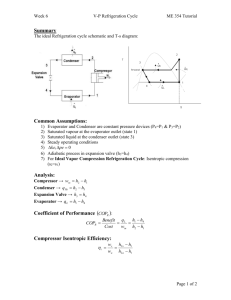
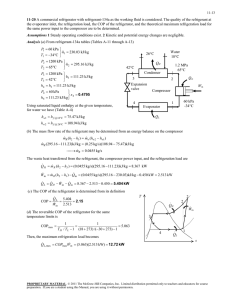
10.2 Analyzing Vapor-Compression Refrigeration Systems 459 T 2s 3 4 1 s Figure 10.4 T–s diagram of an ideal vaporcompression cycle. heat exchangers. If compression occurs without irreversibilities, and stray heat transfer to the surroundings is also ignored, the compression process is isentropic. With these considerations, the vapor-compression refrigeration cycle labeled 1–2s–3–4–1 on the T–s diagram of Fig. 10.4 results. The cycle consists of the following series of processes: Process 1–2s: Isentropic compression of the refrigerant from state 1 to the condenser pressure at state 2s. Process 2s–3: Heat transfer from the refrigerant as it flows at constant pressure through the condenser. The refrigerant exits as a liquid at state 3. Process 3–4: Throttling process from state 3 to a two-phase liquid–vapor mixture at 4. Process 4–1: Heat transfer to the refrigerant as it flows at constant pressure through the evaporator to complete the cycle. All of the processes in the above cycle are internally reversible except for the throttling process. Despite the inclusion of this irreversible process, the cycle is commonly referred to as the ideal vapor-compression cycle. The following example illustrates the application of the first and second laws of thermodynamics along with property data to analyze an ideal vapor-compression cycle. EXAMPLE 10.1 ideal vaporcompression cycle Ideal Vapor-Compression Refrigeration Cycle Refrigerant 134a is the working fluid in an ideal vapor-compression refrigeration cycle that communicates thermally with a cold region at 0C and a warm region at 26C. Saturated vapor enters the compressor at 0C and saturated liquid leaves the condenser at 26C. The mass flow rate of the refrigerant is 0.08 kg/s. Determine (a) the compressor power, in kW, (b) the refrigeration capacity, in tons, (c) the coefficient of performance, and (d) the coefficient of performance of a Carnot refrigeration cycle operating between warm and cold regions at 26 and 0C, respectively. SOLUTION Known: An ideal vapor-compression refrigeration cycle operates with Refrigerant 134a. The states of the refrigerant entering the compressor and leaving the condenser are specified, and the mass flow rate is given. Find: Determine the compressor power, in kW, the refrigeration capacity, in tons, coefficient of performance, and the coefficient of performance of a Carnot vapor refrigeration cycle operating between warm and cold regions at the specified temperatures. 460 Chapter 10 Refrigeration and Heat Pump Systems Schematic and Given Data: Warm region TH = 26°C = 299 K · Qout 3 Condenser 2s T Expansion valve 2s · Wc Compressor 26°C 0°C Evaporator 4 3 Temperature of warm region a 4 1 Temperature of cold region 1 s · Qin Cold region TC = 0°C = 273 K Figure E10.1 Assumptions: 1. Each component of the cycle is analyzed as a control volume at steady state. The control volumes are indicated by dashed lines on the accompanying sketch. 2. Except for the expansion through the valve, which is a throttling process, all processes of the refrigerant are internally reversible. 3. The compressor and expansion valve operate adiabatically. 4. Kinetic and potential energy effects are negligible. 5. Saturated vapor enters the compressor, and saturated liquid leaves the condenser. ❶ Analysis: Let us begin by fixing each of the principal states located on the accompanying schematic and T–s diagrams. At the inlet to the compressor, the refrigerant is a saturated vapor at 0C, so from Table A-10, h1 247.23 kJ/kg and s1 0.9190 kJ/kg # K. The pressure at state 2s is the saturation pressure corresponding to 26C, or p2 6.853 bar. State 2s is fixed by p2 and the fact that the specific entropy is constant for the adiabatic, internally reversible compression process. The refrigerant at state 2s is a superheated vapor with h2s 264.7 kJ/Kg. State 3 is saturated liquid at 26C, so h3 85.75 kJ/kg. The expansion through the valve is a throttling process (assumption 2), so h4 h3. (a) The compressor work input is # # Wc m 1h2s h1 2 # where m is the mass flow rate of refrigerant. Inserting values # 1 kW Wc 10.08 kg/s21264.7 247.232 kJ/kg ` ` 1 kJ/s 1.4 kW 10.2 Analyzing Vapor-Compression Refrigeration Systems 461 (b) The refrigeration capacity is the heat transfer rate to the refrigerant passing through the evaporator. This is given by # # Qin m 1h1 h4 2 10.08 kg/s2|60 s/min|1247.23 85.752 kJ/kg ` 1 ton ` 211 kJ/min 3.67 ton (c) The coefficient of performance is # Qin h1 h4 247.23 85.75 b # 9.24 h2s h1 264.7 247.23 Wc (d) For a Carnot vapor refrigeration cycle operating at TH 299 K and TC 273 K, the coefficient of performance determined from Eq. 10.1 is ❷ bmax ❶ ❷ TC 10.5 TH TC The value for h2s can be obtained by double interpolation in Table A-12 or by using Interactive Thermodynamics: IT. As expected, the ideal vapor-compression cycle has a lower coefficient of performance than a Cornot cycle operating between the temperatures of the warm and cold regions. The smaller value can be attributed to the effects of the external irreversibility associated with desuperheating the refrigerant in the condenser (Process 2s–a on the T–s diagram) and the internal irreversibility of the throttling process. Figure 10.5 illustrates several features exhibited by actual vaporcompression systems. As shown in the figure, the heat transfers between the refrigerant and the warm and cold regions are not accomplished reversibly: the refrigerant temperature in the evaporator is less than the cold region temperature, TC, and the refrigerant temperature in the condenser is greater than the warm region temperature, TH. Such irreversible heat transfers have a significant effect on performance. In particular, the coefficient of performance decreases as the average temperature of the refrigerant in the evaporator decreases and as the average temperature of the refrigerant in the condenser increases. Example 10.2 provides an illustration. ACTUAL CYCLE. T 2s 2 3 Temperature of warm region, TH 4 Temperature of cold region, TC 1 s Figure 10.5 T–s diagram of an actual vapor-compression cycle. 462 Chapter 10 Refrigeration and Heat Pump Systems EXAMPLE 10.2 Effect of Irreversible Heat Transfer on Performance Modify Example 10.1 to allow for temperature differences between the refrigerant and the warm and cold regions as follows. Saturated vapor enters the compressor at 10C. Saturated liquid leaves the condenser at a pressure of 9 bar. Determine for the modified vapor-compression refrigeration cycle (a) the compressor power, in kW, (b) the refrigeration capacity, in tons, (c) the coefficient of performance. Compare results with those of Example 10.1. SOLUTION Known: An ideal vapor-compression refrigeration cycle operates with Refrigerant 134a as the working fluid. The evaporator temperature and condenser pressure are specified, and the mass flow rate is given. Find: Determine the compressor power, in kW, the refrigeration capacity, in tons, and the coefficient of performance. Compare results with those of Example 10.1. Schematic and Given Data: Assumptions: T 2s 3 9 bar 26°C 0°C –10°C 4 1 s 1. Each component of the cycle is analyzed as a control volume at steady state. The control volumes are indicated by dashed lines on the sketch accompanying Example 10.1. 2. Except for the process through the expansion valve, which is a throttling process, all processes of the refrigerant are internally reversible. 3. The compressor and expansion valve operate adiabatically. 4. Kinetic and potential energy effects are negligible. 5. Saturated vapor enters the compressor, and saturated liquid exits the condenser. Figure E10.2 Analysis: Let us begin by fixing each of the principal sates located on the accompanying T–s diagram. Starting at the inlet to the compressor, the refrigerant is a saturated vapor at 10C, so from Table A-10, h1 241.35 kJ/kg and s1 0.9253 kJ/kg # K. The superheated vapor at state 2s is fixed by p2 9 bar and the fact that the specific entropy is constant for the adiabatic, internally reversible compression process. Interpolating in Table A-12 gives h2s 272.39 kJ/kg. State 3 is a saturated liquid at 9 bar, so h3 99.56 kJ/kg. The expansion through the valve is a throttling process; thus, h4 h3. (a) The compressor power input is # # Wc m 1h2s h1 2 # where m is the mass flow rate of refrigerant. Inserting values # 1 kW Wc 10.08 kg/s21272.39 241.352 kJ/kg ` ` 1 kJ/s 2.48 kW (b) The refrigeration capacity is # # Qin m 1h1 h4 2 10.08 kg/s2 060 s/min 0 1241.35 99.562 kJ/kg ` 3.23 ton 1 ton ` 211 kJ/min 10.2 Analyzing Vapor-Compression Refrigeration Systems 463 (c) The coefficient or performance is # Qin h1 h4 241.35 99.56 b # 4.57 h2s h1 272.39 241.35 Wc Comparing the results of the present example with those of Example 10.1, we see that the power input required by the compressor is greater in the present case. Furthermore, the refrigeration capacity and coefficient of performance are smaller in this example than in Example 10.1. This illustrates the considerable influence on performance of irreversible heat transfer between the refrigerant and the cold and warm regions. Referring again to Fig. 10.5, we can identify another key feature of actual vaporcompression system performance. This is the effect of irreversibilities during compression, suggested by the use of a dashed line for the compression process from state 1 to state 2. The dashed line is drawn to show the increase in specific entropy that would accompany an adiabatic irreversible compression. Comparing cycle 1–2–3–4–1 with cycle 1–2s–3–4–1, the refrigeration capacity would be the same for each, but the work input would be greater in the case of irreversible compression than in the ideal cycle. Accordingly, the coefficient of performance of cycle 1–2–3–4–1 is less than that of cycle 1–2s–3–4–1. The effect of irreversible compression can be accounted for by using the isentropic compressor efficiency, which for states designated as in Fig. 10.5 is given by # # 1Wc m2 s h2s h1 hc # # h2 h1 1Wc m2 Additional departures from ideality stem from frictional effects that result in pressure drops as the refrigerant flows through the evaporator, condenser, and piping connecting the various components. These pressure drops are not shown on the T–s diagram of Fig. 10.5 and are ignored in subsequent discussions for simplicity. Finally, two additional features exhibited by actual vapor-compression systems are shown in Fig. 10.5. One is the superheated vapor condition at the evaporator exit (state 1), which differs from the saturated vapor condition shown in Fig. 10.4. Another is the subcooling of the condenser exit state (state 3), which differs from the saturated liquid condition shown in Fig. 10.4. Example 10.3 illustrates the effects of irreversible compression and condenser exit subcooling on the performance of the vapor-compression refrigeration system. EXAMPLE 10.3 Actual Vapor-Compression Refrigeration Cycle Reconsider the vapor-compression refrigeration cycle of Example 10.2, but include in the analysis that the compressor has an efficiency of 80%. Also, let the temperature of the liquid leaving the condenser be 30C. Determine for the modified cycle (a) the compressor power, in kW, (b) the refrigeration capacity, in tons, (c) the coefficient of performance, and (d) the rates of exergy destruction within the compressor and expansion valve, in kW, for T0 299 K (26C). SOLUTION Known: A vapor-compression refrigeration cycle has a compressor efficiency of 80%. Find: Determine the compressor power, in kW, the refrigeration capacity, in tons, the coefficient of performance, and the rates of exergy destruction within the compressor and expansion valve, in kW. 464 Chapter 10 Refrigeration and Heat Pump Systems Schematic and Given Data: Assumptions: T 2 1. Each component of the cycle is analyzed as a control volume at steady state. p2 = 9 bar 2s 2. There are no pressure drops through the evaporator and condenser. 30°C –10°C 3. The compressor operates adiabatically with an efficiency of 80%. The expansion through the valve is a throttling process. 3 T0 = 26°C = 299 K 4 4. Kinetic and potential energy effects are negligible. 5. Saturated vapor at 10C enters the compressor, and liquid at 30C leaves the condenser. 1 6. The environment temperature for calculating exergy is T0 299 K (26C). s Figure E10.3 Analysis: Let us begin by fixing the principal states. State 1 is the same as in Example 10.2, so h1 241.35 kJ/kg and s1 0.9253 kJ/kg # K. Owing to the presence of irreversibilities during the adiabatic compression process, there is an increase in specific entropy from compressor inlet to exit. The state at the compressor exit, state 2, can be fixed using the compressor efficiency # # 1Wc m2 s 1h2s h1 2 hc # # 1h2 h1 2 Wc m where h2s is the specific enthalpy at state 2s, as indicated on the accompanying T–s diagram. From the solution to Example 10.2, h2s 272.39 kJ/kg. Solving for h2 and inserting known values h2 h2s h1 hc h1 1272.39 241.352 10.802 241.35 280.15 kJ/kg State 2 is fixed by the value of specific enthalpy h2 and the pressure, p2 9 bar. Interpolating in Table A-12, the specific entropy is s2 0.9497 kJ/kg # K. The state at the condenser exit, state 3, is in the liquid region. The specific enthalpy is approximated using Eq. 3.14, together with saturated liquid data at 30C, as follows: h3 hf 91.49 kJ/kg. Similarly, with Eq. 6.7, s3 sf 0.3396 kJ/kg # K. The expansion through the valve is a throttling process; thus, h4 h3. The quality and specific entropy at state 4 are, respectively x4 h4 hf4 91.49 36.97 0.2667 hg4 hf4 204.39 and s4 sf4 x4 1sg4 sf4 2 0.1486 10.2667210.9253 0.14862 0.3557 kJ/kg # K (a) The compressor power is # # Wc m 1h2 h1 2 10.08 kg/s2 1280.15 241.352 kJ/kg ` (b) The refrigeration capacity is # # Qin m 1h1 h4 2 1 kW ` 3.1 kW 1 kJ/s 10.08 kg/s2 060 s/min 0 1241.35 91.492 kJ/kg ` 3.41 ton 1 ton ` 211 kJ/min 10.3 Refrigerant Properties (c) The coefficient of performance is ❶ b 1h1 h4 2 1h2 h1 2 1241.35 91.492 1280.15 241.352 465 3.86 (d) The rates of exergy destruction in the compressor and expansion valve can be found by reducing the exergy rate balance # # # or using the relationship Ed T0scv, where scv is the rate of entropy production from an entropy rate balance. With either approach, the rates or exergy destruction for the compressor and valve are, respectively # # # # 1Ed 2 c mT0 1s2 s1 2 and 1Ed 2 valve mT0 1s4 s3 2 Substituting values # kg 1 kW kJ 1Ed 2 c a0.08 b 1299 K2 10.9497 0.92532 # ` ` 0.58 kW s kg K 1 kJ/s ❷ and # 1Ed 2 valve 10.082 1299210.3557 0.33962 0.39 kW ❶ Irreversibilities in the compressor result in an increased compressor power requirement compared to the isentropic compression of Example 10.2. As a consequence, the coefficient of performance is lower. ❷ The exergy destruction rates calculated in part (d) measure the effect of irreversibilities as the refrigerant flows through the compressor and valve. The percentages of the power input (exergy input) to the compressor destroyed in the compressor and valve are 18.7 and 12.6%, respectively. 10.3 Refrigerant Properties From about 1940 to the early 1990s, the most common class of refrigerants used in vaporcompression refrigeration systems was the chlorine-containing CFCs (chlorofluorocarbons). Refrigerant 12 (CCl2F2) is one of these. Owing to concern about the effects of chlorine in refrigerants on the earth’s protective ozone layer, international agreements have been implemented to phase out the use of CFCs. Classes of refrigerants containing various amounts of hydrogen in place of chlorine atoms have been developed that have less potential to deplete atmospheric ozone than do more fully chlorinated ones, such as Refrigerant 12. One such class, the HFCs, contain no chlorine. Refrigerant 134a (CF3CH2F) is the HFC considered by many to be an environmentally acceptable substitute for Refrigerant 12, and Refrigerant 134a has replaced Refrigerant 12 in many applications. Refrigerant 22 (CHClF2) is in the class called HCFCs that contains some hydrogen in place of the chlorine atoms. Although Refrigerant 22 and other HCFCs are widely used today, discussions are under way that will likely result in phasing out their use at some time in the future. Ammonia (NH3), which was widely used in the early development of vaporcompression refrigeration, is again receiving some interest as an alternative to the CFCs because it contains no chlorine. Ammonia is also important in the absorption refrigeration systems discussed in Section 10.5. Hydrocarbons such as propane (C3H8) and methane (CH4) are also under investigation for use as refrigerants. Thermodynamic property data for ammonia, propane, and Refrigerants 22 and 134a are included in the appendix tables. These data allow us to study refrigeration and heat pump systems in common use and to investigate some of the effects on refrigeration cycles of using alternative working fluids. A thermodynamic property diagram widely used in the refrigeration field is the pressure–enthalpy or p–h diagram. Figure 10.6 shows the main features of such a property p–h diagram 10.7 Gas Refrigeration Systems 475 the work developed by the turbine of a Brayton refrigeration cycle is significant relative to the compressor work input. The heat transfer from the cold region to the refrigerant gas circulating through the lowpressure heat exchanger, the refrigeration effect, is # Qin # h1 h4 m The coefficient of performance is the ratio of the refrigeration effect to the net work input: # # 1h1 h4 2 Qin m # # b # # (10.11) 1h h Wc m Wt m 2 1 2 1h3 h4 2 In the next example, we illustrate the analysis of an ideal Brayton refrigeration cycle. EXAMPLE 10.4 Ideal Brayton Refrigeration Cycle Air enters the compressor of an ideal Brayton refrigeration cycle at 1 bar, 270K, with a volumetric flow rate of 1.4 m3/s. If the compressor pressure ratio is 3 and the turbine inlet temperature is 300K, determine (a) the net power input, in kW, (b) the refrigeration capacity, in kW, (c) the coefficient of performance. SOLUTION Known: An ideal Brayton refrigeration cycle operates with air. Compressor inlet conditions, the turbine inlet temperature, and the compressor pressure ratio are given. Find: Determine the net power input, in kW, the refrigeration capacity, in kW, and the coefficient of performance. Schematic and Given Data: · Qout T3 = 300K Heat exchanger T 2s 2s 3 Turbine Compressor p = 3 bars · Wcycle T3 = 300K 3 p = 1 bar Heat exchanger 4s · Qin 1 1 (AV)1 = 1.4 m3/s T1 = 270K p1 = 1 bar T1 = 270K 4s s Figure E10.4 Assumptions: 1. Each component of the cycle is analyzed as a control volume at steady state. The control volumes are indicated by dashed lines on the accompanying sketch. 2. The turbine and compressor processes are isentropic. 476 Chapter 10 Refrigeration and Heat Pump Systems 3. There are no pressure drops through the heat exchangers. 4. Kinetic and potential energy effects are negligible. 5. The working fluid is air modeled as an ideal gas. Analysis: The analysis begins by determining the specific enthalpy at each numbered state of the cycle. At state 1, the temperature is 270 K. From Table A-22, h1 270.11 kJ/kg, pr1 0.9590. Since the compressor process is isentropic, h2s can be determined by first evaluating pr at state 2s. That is pr2 p2 p 13210.95902 2.877 p1 r1 Then, interpolating in Table A-22, we get h2s 370.1 kJ/kg. The temperature at state 3 is given as T3 300 K. From Table A-22, h3 300.19 kJ/kg, pr3 1.3860. The specific enthalpy at state 4s is found by using the isentropic relation pr4 pr3 p4 11.38602 1132 0.462 p3 Interpolating in Table A-22, we obtain h4s 219.0 kJ/kg. (a) The net power input is # # Wcycle m 3 1h2s h1 2 1h3 h4s 2 4 # This requires the mass flow rate m, which can be determined from the volumetric flow rate and the specific volume at the compressor inlet: 1AV2 1 # m v1 Since v1 (RM )T1p1 1AV2 1p1 # m 1RM2T1 a 11.4 m3/s2 11 bar2 8.314 kJ b 1270 K2 28.97 a 105 N/m2 1 kJ ba 3 # b 1 bar 10 N m 1.807 kg/s Finally # 1 kW b Wcycle 11.807 kg/s2 3 1370.1 270.112 1300.19 219.02 4 kJ/kg a 1 kJ/s 33.97 kW (b) The refrigeration capacity is # # Qin m 1h1 h4s 2 11.807 kg/s21270.11 2192 kJ/kg a 1 kW b 1 kJ/s 92.36 kW (c) The coefficient of performance is # Qin 92.36 b # 2.72 33.97 Wcycle Irreversibilities within the compressor and turbine serve to decrease the coefficient of performance significantly from that of the corresponding ideal cycle because the compressor work requirement is increased and the turbine work output is decreased. This is illustrated in the example to follow. 10.7 Gas Refrigeration Systems EXAMPLE 10.5 477 Brayton Refrigeration Cycle with Irreversibilities Reconsider Example 10.4, but include in the analysis that the compressor and turbine each have an isentropic efficiency of 80%. Determine for the modified cycle (a) the net power input, in kW, (b) the refrigeration capacity, in kW, (c) the coefficient of performance, and interpret its value. SOLUTION Known: A Brayton refrigeration cycle operates with air. Compressor inlet conditions, the turbine inlet temperature, and the compressor pressure ration are given. The compressor and turbine each have an efficiency of 80%. Find: Determine the net power input and the refrigeration capacity, each in kW. Also, determine the coefficient of performance and interpret its value. Schematic and Given Data: 2 T 2s Assumptions: p = 3 bars 3 1. Each component of the cycle is analyzed as a control volume at steady state. 2. The compressor and turbine are adiabatic. T3 = 300 K 1 3. There are no pressure drops through the heat exchangers. T1 = 270 K 4. Kinetic and potential energy effects are negligible. p = 1 bar 5. The working fluid is air modeled as an ideal gas. 4 4s s Figure E10.5 Analysis: (a) The power input to the compressor is evaluated using the isentropic compressor efficiency, c. That is # # # 1Wc m2 s Wc # hc m # # The value of the work per unit mass for the isentropic compression, (Wcm)s, is determined with data from the solution in Example 10.4 as 99.99 kJ/kg. The actual power required is then # # # m 1Wcm2 s 11.807 kg/s2 199.99 kJ/kg2 # Wc hc 10.82 225.9 kW turbine # The # # power output is determined in a similar manner, using # # the turbine isentropic efficiency t. Thus, # Wt m ht(Wt m)s. Using data form the solution to Example 10.4 gives (Wt m)s 81.19 kJ /kg. The actual turbine work is then # # # # Wt m ht 1Wt m2 s 11.807 kg/s2 10.82 181.19 kJ/kg2 117.4 kW The net power input to the cycle is # Wcycle 225.9 117.4 108.5 kW 478 Chapter 10 Refrigeration and Heat Pump Systems (b) The specific enthalpy at the turbine exit, h4, is required # # to# evaluate the refrigeration capacity. This enthalpy can be deter# mined by solving Wt m(h3 h4) to obtain h4 h3 Wt m. Inserting known values h4 300.19 a 117.4 b 235.2 kJ/kg 1.807 The refrigeration capacity is then # # Qin m 1h1 h4 2 11.8072 1270.11 235.22 63.08 kW (c) The coefficient of performance is # Qin 63.08 b # 0.581 108.5 Wcycle The value of the coefficient of performance in this case is less than unity. This means that the refrigeration effect is smaller than the net work required to achieve it. Additionally, note that irreversibilities in the compressor and turbine have a significant effect on the performance of gas refrigeration systems. This is brought out by comparing the results of the present example with those of Example 10.4. Irreversibilities result in an increase in the work of compression and a reduction in the work output of the turbine. The refrigeration capacity is also reduced. The overall effect is that the coefficient of performance is decreased significantly. ADDITIONAL GAS REFRIGERATION APPLICATIONS To obtain even moderate refrigeration capacities with the Brayton refrigeration cycle, equipment capable of achieving relatively high pressures and volumetric flow rates is needed. For most applications involving air conditioning and for ordinary refrigeration processes, vaporcompression systems can be built more cheaply and can operate with higher coefficients of performance than gas refrigeration systems. With suitable modifications, however, gas refrigeration systems can be used to achieve temperatures of about 150C, which are well below the temperatures normally obtained with vapor systems. Figure 10.14 shows the schematic and T–s diagram of an ideal Brayton cycle modified by the introduction of a regenerative heat exchanger. The heat exchanger allows the air entering the turbine at state 3 to be cooled below the warm region temperature TH. In the subsequent expansion through the turbine, the air achieves a much lower temperature at state 4 than would have been possible without the regenerative heat exchanger. Accordingly, the refrigeration effect, achieved from state 4 to state b, occurs at a correspondingly lower average temperature. T · Qin 2 Heat exchanger b TH 4 3 · Qout a 1 a 3 1 2 b · Wcycle Turbine Figure 10.14 4 Compressor Brayton refrigeration cycle with a regenerative heat exchanger. s