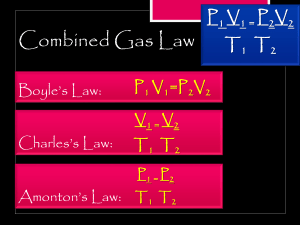
Science 10 – 4th Quarter Ms. Jonessa U. Gurrea Name: ____________________________ Grade/Section: _________________________ PROBLEM SET NO. 1: BOYLE’S LAW Abbreviations atm - atmosphere mm Hg - millimeters of mercury torr - another name for mm Hg 1 atm =101325 pascal (Pa). 1 atm = 760 mmHg 1 atm = 760 Torr 1mmHg = 1 Torr Boyle’s Law Formula P1V1 = P2V2 0°C + 273.15 = 273.15K (0°C × 9/5) + 32 = 32°F (32°F − 32) × 5/9 = 0°C 1 L = 1000mL Note: Standard Temperature and Pressure (STP) involving gases: Temperature: 0 degrees Celsius (273.15 degrees Kelvin or 32 degrees Fahrenheit) Pressure: 1 atm (101.325 kilopascal or 760 Torr). Example 1: 2.00 L of a gas is at 740.0 mmHg pressure. What is its volume at 760 mmHg pressure? Answer: this problem is solved by inserting values into P1V1 = P2V2. (740.0 mmHg) (2.00 L) = (760.0 mmHg) (x) Example #1: 2.00 L of a gas is at 740.0 mmHg pressure. What is its volume at standard pressure? Answer: this problem is solved by inserting values into P1V1 = P2V2. (740.0 mmHg) (2.00 L) = (760.0 mmHg) (x) Directions: Solve the following problems using the AGSA method (Ask, Given, Solution, Answer). Show your answers neatly and completely. 1. A gas occupies 12.3 liters at a pressure of 40.0 mm Hg. What is the volume when the pressure is increased to 60.0 mm Hg? 2. If a gas at 25.0 °C occupies 3.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm? 3. A gas occupies 1.56 L at 1.00 atm. What will be the volume of this gas if the pressure becomes 3.00 atm? 4. A gas occupies 11.2 liters at 0.860 atm. What is the pressure if the volume becomes 15.0 L? 5. 500.0 mL of gas is collected at 745.0 mm Hg. What will the volume be at standard pressure? 6. Convert 350.0 mL at 740.0 mm of Hg to its new volume at standard pressure. 7. If a gas at 25.0 °C occupies 3.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm? 8. To what pressure must a gas be compressed in order to get into a 3.00 cubic foot tank the entire weight of a gas that occupies 400.0 cu. ft. at standard pressure? 9. A gas occupies 1.56 L at 1.00 atm. What will be the volume of this gas if the pressure becomes 3.00 atm? 10. A gas occupies 11.2 liters at 0.860 atm. What is the pressure if the volume becomes 15.0 L? PROBLEM SET NO. 2: CHARLES’ LAW Directions: Solve the following problems using the AGSA method (Ask, Given, Solution, Answer). Show your answers neatly and completely. Convert all temperature units into Kelvin (K). Charles Law Formula: V1T1=V2T2 1) A 50.0 ml soap bubble is blown in a 27.0°C room. It drifts out an open window and lands in a snow bank at 3.0°C. What is its new volume? 2) A balloon was inflated to a volume of 5.0 liters at a temperature of 7.0°C. It landed in an oven and was heated to 147°C. What is its new volume? 3) During the day at 27°C a cylinder with a sliding top contains 20.0 liters of air. At night it only holds 19 liters. What is the temperature at night? Give the answer in Kelvin and °C? 4) A 113L sample of Helium at 27°C is cooled to -78°C. Calculate the new volume of the Helium. 5. A 0.20 ml CO2 bubble in a cake batter is at 27°C. In the oven it gets heated to 177°C. What is its new volume? 6. A 500.0 ml. Glass filled with air is placed into water up-side-down while at 7.0°C. The water is heated to 77°C. How much air bubbles out from under the glass? 7. The temperature inside my refrigerator is about 40 Celsius. If I place a balloon in my fridge that initially has a temperature of 220 C and a volume of 0.5 liters, what will be the volume of the balloon when it is fully cooled by my refrigerator? 8. A man heats a balloon in the oven. If the balloon initially has a volume of 0.4 liters and a temperature of 20 0 C, what will the volume of the balloon be after he heats it to a temperature of 250 0 C? 9. How hot will a 2.3 L balloon have to get to expand to a volume of 400 L? Assume that the initial temperature of the balloon is 25 0 C. 10. Calculate the decrease in temperature (in Celsius) when 2.00 L at 21.0 °C is compressed to 1.00 PROBLEM SET NO. 3: GAY-LUSSAC’S LAW Directions: Solve the following problems using the AGSA method (Ask, Given, Solution, Answer). Show your answers neatly and completely. Convert all temperature units into Kelvin (K). Gay-Lussac’s Law: P1/T1=P2/T2. 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20°C to 30 °C. 2. A gas has a pressure of 0.370 atm at 50.0 °C. What is the pressure at standard temperature? 3. A gas has a pressure of 699.0 mm Hg at 40.0 °C. What is the temperature at standard pressure? 4. If a gas is cooled from 323.0 K to 273.15 K and the volume is kept constant what final pressure would result if the original pressure was 750.0 mm Hg? 5. If a gas in a closed container is pressurized from 15.0 atmospheres to 16.0 atmospheres and its original temperature was 25.0 °C, what would the final temperature of the gas be? 6. A 30.0 L sample of nitrogen inside a rigid, metal container at 20.0 °C is placed inside an oven whose temperature is 50.0 °C. The pressure inside the container at 20.0 °C was at 3.00 atm. What is the pressure of the nitrogen after its temperature is increased? 7. A rigid container has an initial pressure of 1.50 atm at 21 C. What will the pressure be if the temperature is increase to 121 C? 8. The pressure inside a container s 770 mmHg at a temperature of 57 C. What would the pressure be at 75 C? 9. A rigid container is at a temperature of 112 C. When heated to 224 C, the pressure was 288 kPa. What was the initial pressure? 10. Calculate the final pressure inside a scuba tank after it cools from 1.00 x 10³ °C to 25.0 °C. The initial pressure in the tank is 130.0 atm.