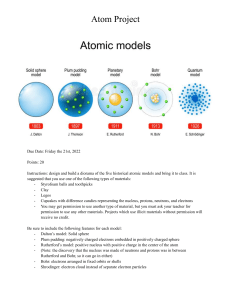
Damon Wang July 22nd, 2020 HISTORY OF THE ATOM 460 BC Democritus develops the idea of atoms He pounded up materials in his pestle and mortar until he had reduced them to smaller and smaller particles which he called ATOMA (greek for indivisible) HISTORY OF THE ATOM 1808 John Dalton All matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ATOMS Dalton’s Atomic Theory 1. All matter consists of tiny particles, atoms. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. ... HISTORY OF THE ATOM 1898 Joseph John Thompson Found that atoms could sometimes eject a far smaller negative charged particle which he called an ELECTRON HISTORY OF THE ATOM 1904 Atom was made up of electrons scattered unevenly within an elastic sphere surrounded by a soup of positive charge to balance the electron's charge like plums surrounded by pudding. PLUM PUDDING MODEL HISTORY OF THE ATOM 1910 Ernest Rutherford Fired Helium nuclei at a piece of gold foil which was only a few atoms thick. Although most of them passed through. About 1 in 10,000 hits Rutherford’s Apparatus Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work in nuclear chemistry. beam of alpha particles radioactive substance circular ZnS - coated fluorescent screen gold foil HISTORY OF THE ATOM New evidence allowed him to propose a more detailed model with a central nucleus. Positive charge was all in a central nucleus. With this holding the electrons in place by electrical attraction However, this was not the end of the story. HISTORY OF THE ATOM 1913 Niels Bohr Studied under Rutherford at the Victoria University in Manchester. Bohr refined Rutherford's that the electrons were in orbits. Rather like planets orbiting the sun, each orbit is only able to contain a set number of electrons. Bohr’s Atom electrons in orbits nucleus Models of the Atom Greek Dalton’s model model (400 B.C.) (1803) Thomson’s plum-pudding model (1897) Bohr’s model (1913) Rutherford’s model (1909) Charge-cloud model (present) Structure of the Atom There are two regions The nucleus • With protons and neutrons – Positive charge(protons) – Almost all the mass Electron cloud – Most of the volume of an atom – The region where the electron can be found Size of an atom • Atoms are incredibly tiny. • Measured in picometers (10-12 meters) – H atom, 32 pm radius • Nucleus tiny compared to atom – Radius near 10-15 m. – Density near 1014 g/cm3 • If the atom was the size of a stadium, the nucleus would be the size of a marble. HELIUM ATOM Shell proton + electron N N + - neutron Subatomic particles Name Symbol Relative Charge mass Actual mass (g) Electron e- -1 1/1840 9.11 x 10-28 Proton p+ +1 1 1.67 x 10-24 Neutron no 0 1 1.67 x 10-24 Symbols Contain the symbol of the element, the mass number and the atomic number # protons + # neutrons mass number # protons Mass number Atomic number X Symbols • Find the – number of protons = 9 + – number of neutrons = 10 – number of electrons = 9 – Atomic number = 9 – Mass number = 19 19 9 F SUMMARY 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus. 3. The number of Protons = Number of Electrons. 4. Electrons orbit the nucleus in shells.