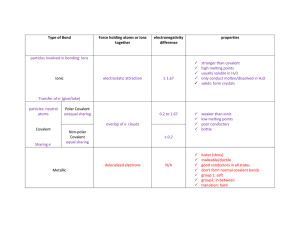
Trends in the Periodic Table Lesson objectives • Recognise periodicity in the table and be able to account for this in terms of effective nuclear charge, ionisation energy, bond types etc. • Recall and be able to explain general trends in melting and boiling points, and acid-base behaviour (the above using the elements Na to Ar as exemplification) • Recognise and be able to account in terms of effective nuclear charge for trends down groups: bond types, melting and boiling points, reactivity etc. (the above using (as a minimum) groups 1 and 17 as exemplification) Success criteria • Describe and explain the trends in melting point of period 3 elements, group 1 and 7 elements. • Describe and explain the trends in reactivity of group 1 and 7 elements. Keywords • • • • • • • • • Metallic bond Covalent bond Metallic lattice Charge density Covalent structures Giant molecular Simple molecular Monoatomic Reactivity Period 3 melting points trends Describe all the trends you can see in the graph. Can you explain this trend in terms of the bonding and structure of the elements? Melting points across period 3 elements • They are based on the bonding and structure present. • Metallic bond and metallic structure • Na, Mg and Al • Covalent bond and giant molecular structure • Si • Covalent bond and simple molecular structure • P, S, Cl, Ar- No bonding Structure and Bonding A summary of the variations in structure and bonding of elements across Periods 3 Increase in melting point from Na to Al From sodium to aluminium: •The positive charge increases and the ionic size decreases •The number of are delocalised atom size. increases. The ions drawn to electrons reflect theirper relative •This results in a higher charge density(larger charge and smaller size of ions gives rise to a larger charge density) •This increases the strength of the metallic bond which in turn increases the melting and boiling points. MELTING POINT TREND - NON METALS relative mass melting point / K Covalent macromolecular P4 124 317 S8 256 392 Cl2 71 172 Ar 40 84 Covalent molecular Covalent molecular Covalent molecular Covalent atomic • Explanation for covalent structures • Silicon has a giant covalent structure in which its atoms are joined by strong covalent bonds. • There are weak van der Waals forces between the molecules of phosphorus, sulphur, chlorine and Argon. • This force is more in sulfur (S8) due to increased molecular mass followed by phosphorous(P4), chlorine(Cl2) and Argon which exist as single atoms (Ar) Trends in melting and boiling points of halogens Melting and boiling of simple covalent molecules involves breaking the intermolecular force that hold the molecules together Melting points and boiling points increase down the group. Trends in melting and boiling points of halogens Going down the group, halogen molecules increase in size and the number of electrons increase. This leads to greater van der Waals forces between molecules This increases the energy needed to separate the molecules and therefore higher melting and boiling points going down the group van der Waals forces fluorine atomic radius = 42 × 10-12 m boiling point = -118 °C iodine atomic radius = 115 × 10-12 m boiling point = 184 °C Trends in melting and boiling point of alkali metals Melting and boiling of metals involves the breaking the force of attraction between the metal ions and the delocalized electrons (metallic bond) Melting points and boiling points decrease down the group. • • Going down the group, the atoms become larger This makes the attraction between the delocalised electron and the nuclei weaker. Hence less energy is needed down the group Reactivity • To react the atom must form an ion • For metals They react by losing electrons e.g K(g) K+(g) + e• For non-metals React by gaining electrons e.g Cl2(g) + 2e- 2Cl-(g) Reactivity of halogens F The atoms of each element get larger going down the group. This means that the outer shell gets further away from the nucleus and is shielded by more electron shells. The further the outer shell is from the positive attraction of the nucleus, the harder it is to attract another electron to complete the outer shell. This is why the reactivity of the halogens decreases going down group 7. Cl Br decrease in reactivity The reactivity of halogens decreases going down the group. What is the reason for this? Reactivity of alkali metals • Metals tend to be have low ionization energy. This means outer electrons are easily removed. Li Na • Form ions by losing their outermost electrons K • Reactivity increase down the group Rb Cs Increasing Reactivity Reactivity of Alkali Metals – This is caused by increase in Li atomic size and shielding – Hence the outer electrons are easier to lose down the group. – This means that reactivity increases with the size of the atom. – Metallic character also increases down the group due to the ease with which electrons are lost Na 3P 4N 11P 12N Reactions of period 3 elements with oxygen Element Description Equation Na burns vigorously with a yellow flame to form a white solid burns vigorously with a bright white flame to form a white solid 4Na(s) + O2(g) → 2Na2O(s) Al burns vigorously with a bright white flame to form a white solid 4Al(s) + 3O2(g) → 2Al2O3(s) Si burns with a bright white flame and white smoke to form a white solid Si(s) + O2(g) → SiO2(s) P burns spontaneously with a bright white flame and smoke to form a white solid burns with a blue flame to form a colourless gas P4(s) + 5O2(g) → P4O10( excess) Mg S 2Mg(s) + O2(g) → 2MgO(s) S(s) + O2(g) → SO2(g) Other oxides P: if oxygen is limited, phosphorus(III) oxide can also form: P4(s) + 3O2(g) → P4O6(s). S: some sulfur trioxide can also form: 2S(s) + 3O2(g) → 2SO3(g) Chlorine doesn’t react directly with oxygen but has its 3 oxides i.e Cl20, Cl2O5 and Cl2O7 Argon is unreactive. Bonding of period 3 oxides • Period 3 elements react with oxygen to form oxides • Some have more than one oxides e.g P,S and Cl • Atoms of lower electronegativity lose electrons to the oxygen atoms • Atoms of higher electronegativity share electrons with the oxygen atoms • Because of this, bonding of the oxides of elements across period 3 becomes less ionic and more covalent Bonding and structure in period 3 oxides • • • • Ionic bond and giant ionic structures Na2OMgO Al2O3 – It has some degree of covalent character • • • • • Covalent bond SiO2 - giant covalent structure P4O10 – simple covalent SO2/SO3 –simple covalent Cl2O/Cl2O5/Cl2O7 -simple covalent Acid-base properties of the oxides of period 3 • Due to the variation in nature of bonding of the oxides, they show different behavior towards water, acids and bases • • • • Behaviour towards acids and bases Basic oxides Amphoteric- reacts with both acids and bases Acidic oxides Basic oxides • Na2O and MgO are ionic • Na2O is highly soluble while MgO is slightly soluble in water • They react with water to form hydroxides • Na2O(s) + H2O(l) 2NaOH(aq) (vigorous) pH 12-14 (strongly alkaline) • • • • • MgO(s) + H2O(l) Mg(OH)2(aq) pH 9-10 (weakly alkaline) Moreover, they react with acids to form salt and water Na2O(s) + 2HCl(l) 2NaCl(aq)+ H2O(l) Thus they show basic properties and are basic oxides Amphoteric oxides • • • • Al2O3 is ionic oxide with covalent character It is insoluble in water It reacts with both acids and bases- amphoteric Reactions with acid • Reaction with base Acidic oxides • Silicon dioxide, SiO2, phosphorous pentoxide, P4O10, sulfur dioxide, SO2, and dichloride monoxide, Cl2O are covalent • All, except SiO2 react with water to form acids. SiO2 is a giant covalent molecule • P4O10(s) + 6H2O(l) 4H3PO4 (aq) • SO2(g) + H2O(l) H2SO3(aq) • Furthermore, they react with dilute alkali to form salt and water e.g • Hence, they show acidic properties and are acidic oxides Summary Oxides Na2O MgO Al2O3 SiO2 P4O10 SO2/SO 3 Bonding ionic ionic structure Giant ionic Giant ionic Ionic with covalent character Giant Ionic Covalent Covalent Covalent Aci/base Basic property Basic amphoteric Acidic Simple covalent Simple covalent Simple covalent Acidic Acidic