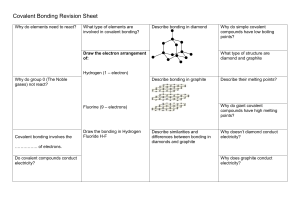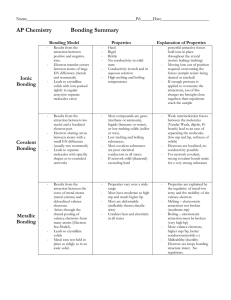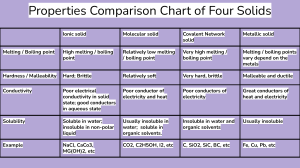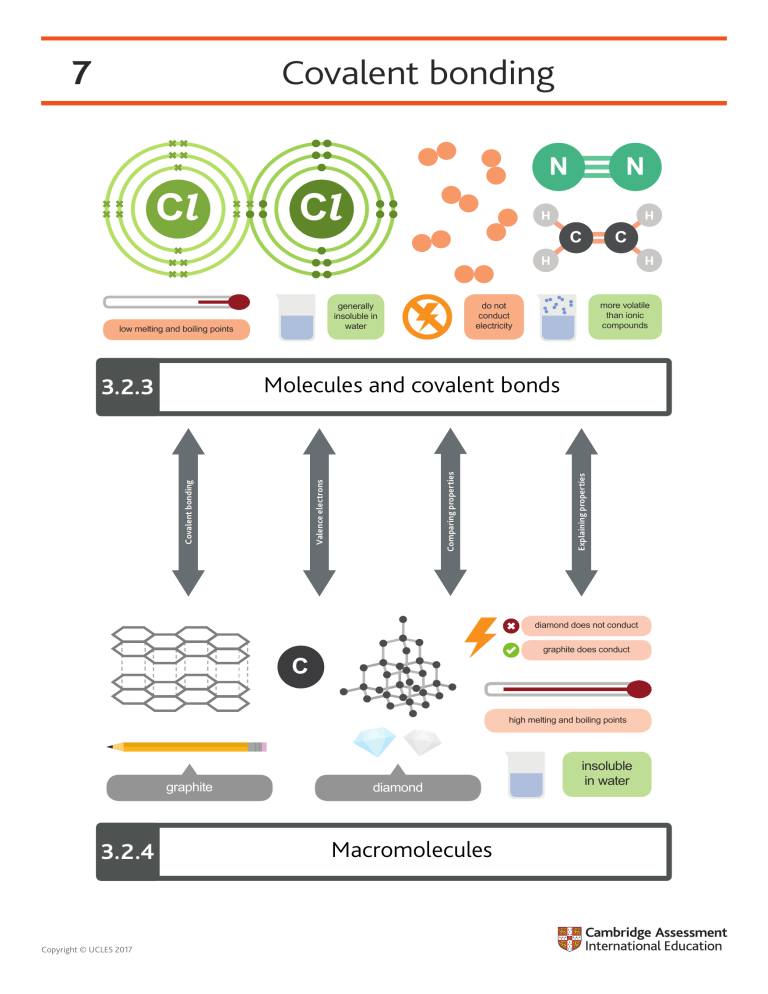
7 Covalent bonding Cl H C more volatile than ionic compounds do not conduct electricity generally insoluble in water C H H H low melting and boiling points N H Cl N Explaining properties Covalent bonding Valence electrons Comparing properties Molecules and covalent bonds 3.2.3 diamond does not conduct graphite does conduct C high melting and boiling points graphite 3.2.4 Copyright © UCLES 2017 diamond Macromolecules insoluble in water