Unit 7: Energy Jan. 2016 10.1
advertisement

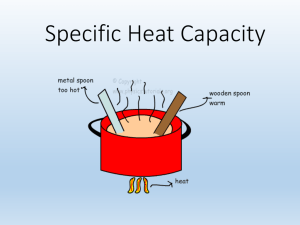
Unit 7: Energy Jan. 2016 10.1 Energy: The capacity to do work o Potential Energy (PE) : resting/ stored energy o Kinetic Energy (KE) : Energy of motion KE = mv2 Law of conservation of energy: Energy cannot be created nor destroyed only converted into different forms. Temperature: Average Kinetic energy. Heat (Q): The flow of thermal energy between two objects when there is a difference in temperature. o Flows from hot to cold, more energy to less energy State Function: the end result is INDEPENDENT of the path taken System: What is being studied by the observer Surroundings: Everything else Endothermic: When energy flows into the system o Q > 0 (greater than zero) Exothermic: Energy flowing out of the system o Q < 0 (less than zero) 10.2 Units for heat: o Joules (J) – SI unit for energy o Calories (cal) : the amount of energy requires to raise ONE GRAM ONE degree Celsius o BTU: British Thermal Units 1 calories = 4.184 Joules Specific Heat Capacity: the amount of energy requires to raise ONE GRAM of a substance ONE degree Celsius o For water it is 4.184 J/ g C or 1.0 cal/ g C o Specific to each substance o Used to determined what the substance can be used for o Q= (Cp)(m)(ΔT) Q= heat Cp = specific heat capacity Can also be indicated as S m = mass (in grams) ΔT = TF - TI Change in temperature