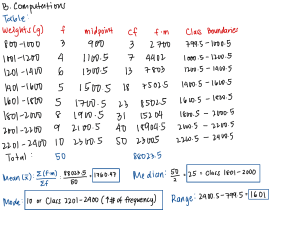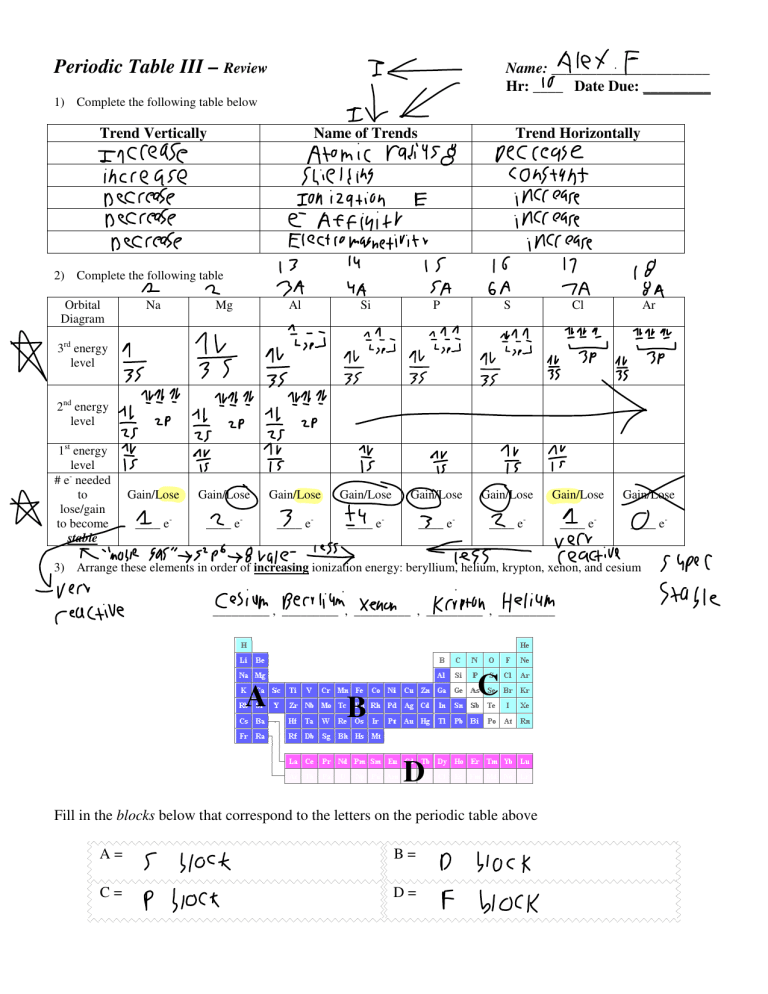
Alex F Periodic Table III – Review It 1) Complete the following table below Trend Vertically Name of Trends Increase Ionization E E Affinito 3rd energy level nd I 2 energy level 1st energy level # e- needed to lose/gain to become stable sa ta rMg Al Gain/Lose a ____ e- sa Ta Si P S ta É Is 03 QQ Gain/Lose Gain/Lose Gain/Lose Gain/Lose ____ e- ____ e- ____ e- ____ e- sasssipbirate Ea Ar Cl É it É increase increase increase Electromasnetivitr 2) Complete the following table Na Decrease constant shielding Decrease Decrease Decrease a Trend Horizontally Atomic rating increase Orbital Diagram Name: _____________________ Hr: ____ 10 Date Due: _________ F Q Gain/Lose Gain/Lose ____ e- ____ e- Is a Yeractive ____ e- 3) Arrangeone these elements in order of increasing ionization energy: beryllium, helium, krypton, xenon, and cesium ven reactive cesium Kripton, Helium _________ , Berilium _________ , xenon _________ , _________ _________ A C B D Fill in the blocks below that correspond to the letters on the periodic table above A= C= S block B= D block P block D= F block I Gain/Lose super Stable B C D A Fill in the regions below that correspond to the letters on the periodic table above A= C= B= metals D= Non meta's metalloils Noble gases Fill in the groups below that correspond to the letters on the periodic table above A= Hidrogen group G= Nitrogen Group Alkaline metals H= oxygen group C= Alkaline earth metals I= Halogenes D= J= noble gases B= E= F= Transition metals Boron group carton group Larger Atomic Radius? Li or N Co or Ir Rb or I C or Br Na or Be Mn or Cs 80 80 Smaller Atomic Radius F- or Na+ Sr2+ or K+ N3- or S2I- or Ca2+ O Al3+ or B3+ Cl- or I- 8 00 K= Lanthiciles L= Actinides Larger Ionization Energy? F or Ba Ca or Al K or Ar Cl or Cs N or Bi Sr or Xe 08 80 Smaller Electron Affinity? I or Cl K or Ca B or Pb Mg or Br C or I Na or Cs 8 O 80 Larger Electronegativity? Rb or Sn Be or Ba Al or Br N or S Si or Fr Fe or Au 00 O of BUILDING THE PERIODIC TABLE The following elements belong together in families as grouped below. The elements listed are not necessarily in order. The letters are not the symbols for the elements. ZRD, SIFP, JXBE, LHT, QKA, WOV, YMC, GUN The assignment is to arrange these elements in the proper periodic form, according to the information given below. Fill in the answers in the periodic table provided at the bottom of this page. Use your periodic table for assistance if necessary. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. U has a total of six electrons. (Used as an example below – U is carbon, therefore G and N are either silicon or germanium.) A is the second most common element in the atmosphere. E is a noble gas. S is an alkali metal. O is a halogen. O has an atomic number larger than V but smaller than W. The charge on an L ion is 2+. C has five electrons in its outer energy level. The atomic mass of T is more than that of H but less than that of L. M has an atomic number one less than that of A. The electrons of atom N are distributed in three energy levels. R has the largest atomic mass of its group. F is a gas at room temperature. Atom B contains 10 protons. Q has an atomic mass less than that of K. Y is more metallic than either M or C. X has an atomic number one higher than F. D has the smallest atomic mass in its group. P is the most reactive element in its family. J has the greatest density of the elements in its group as listed. Atoms of I are larger than those of S. I F S p VIII II I III V U T SIFPLAT IV the dotted lines provide a workspace for listing the families R ere G GUN ZRD L VI I y É VII K W CMV QKA WOV TYRE The successive ionization energies for one of the period three elements is listed below. Which element is referred to? a) Na b) Mg c) Al d) Si e) P a) Na b) Mg c) Al d) Si e) P E1 577.4 kJ/mol E1 496 kJ/mol E2 1,816 kJ/mol E2 4,562 kJ/mol Explain your reasoning Explain your reasoning E3 2,744 kJ/mol E3 6,912 kJ/mol E4 11,580 kJ/mol E4 9,544 kJ/mol E5 15,030 kJ/mol E5 13,353 kJ/mol O onto Al Has 3 Valence electrons Onto Na Has valence electrons 2