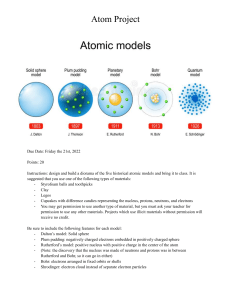
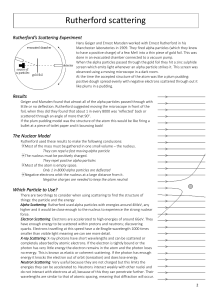
Rutherford's Atomic Model Worksheet
advertisement

Fill in the blanks with the correct answer based on the given options. mass deflected nuclear alpha particles nucleus 1. In Rutherford’s experiment, a visible flash detected by the fluorescent screen signifies the arrival of _________. 2. Rutherford’s experiment led him to propose the ________ model of the atom. 3. According to Rutherford’s model, the atom has a small massive core called the _________. 4. The discovery of the nucleus, the heavy, positively charged body at the center or nucleus took place when the alpha particles________ backward. 5. The _______ of the atom is concentrated in the nucleus.