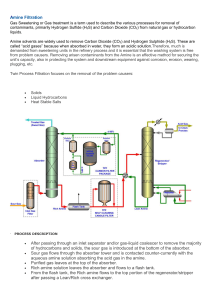
Special Focus Process Optimization D. B. ENGEL, Nexo Solutions, Amine Optimization Division, The Woodlands, Texas; and S. NORTHROP, ExxonMobil Upstream Research, Spring, Texas Managing process contaminants in amine treating units—Part 1: Lean amine filtration drocarbons). Fouling often takes place at high temperatures and/ Installation and operation of suitable contaminant removal or low-velocity fluid locations, such as heat exchangers, columns systems, such as filtration, coalescing and adsorption equipment, with trays (less common) and packing (more common). have become key components in amine units. These systems reFouling or deposits may have various formation mechamove or avoid contaminants that negatively affect the process and nisms. However, it is generally accepted that they can originate its equipment. They also enable the unit to operate with stability, from polymerization reactions by light, heat, free radicals (e.g., reliability and low operational costs, while achieving design profrom oxygen), condensation of dissolved components produccessing capacity. Typical contaminant removal systems for amine ing insoluble materials, or the settling of solids (or immiscible units consist of inlet separators, gas demisters or knockout drums, liquids) in suspension. liquid filters, activated carbon beds and gas coalescers. Additional Today, some types of fouling or deposition can be minitechnologies, such as liquid coalescers for rich amine streams to mized by using chemical means, such as dispersants, oxygen remove emulsified hydrocarbons, and amine recovery systems scavengers, free radical inhibitors or condensation reaction infor treated streams, are increasingly used to protect and mainhibitors. Filtration and/or adsorption methods can be used to tain amine unit performance and recover often expensive amine minimize deposition mechanisms, such as precipitation and/or solvents while protecting units downstream. polymerization and condensation reactions. Both filtration and Contaminant removal systems give amine units a greater toladsorption are effective, depending on the process conditions erance to process upsets. Without these systems, well-designed and the type of contaminant causing fouling or depositions. and properly operated amine units can suffer from several inefBoth fouling and deposits can lead to detrimental situations in ficiencies and problems, such as high operating costs, low efamine units, such as energy losses, inefficiency of heat exchangficiency of H2S and CO2 removal, amine solvent losses, amine ers, column packing clogging and under-deposit corrosion. degradation and excessive maintenance. Fouling can significantly shorten the online life of filters and/ Amine units may experience a variety of challanges, but or retention times in settling tanks, if they are not cleaned. FIG. 2 most of these issues come down to a few core problems. Design limitations, contaminant removal efficiencies or incorrect operillustrates solids deposition in column packing. ational parameters are usually the central problems found in most amine units. The Hydrocarbons in acid gas most common amine unit difficulties are Amine carryover Cooling Acid gas shown in FIG. 1. Lean amine suspended solids Treated gas/liquid Dissolved contaminants Contaminant removal plays a significant Heat exchanger fouling role in amine unit operation and perforPoor temperature control Cooling mance. Filtration and separation technoloReflux Treated feed gas or Lean amine accumulator gies can considerably reduce or eliminate liquid not meeting specification the variety of problems frequently found in Foaming Regenerator amine units, as indicated in FIG. 1. Several Surge Contactor Corrosion tank (absorber) of the problems amine units experience Fouling Foaming Lean/rich Poor regeneration throughout their lifetime can be addressed Corrosion exchanger Fouling with proper filtration and separation, as de- Feed gas/liquid Poor contactor performance Reboiler scribed in the following sections. Flash gas Amine unit problems: Fouling and deposition. Fouling is a process where solids physically or chemically accumulate on the surfaces of process equipment, and it is caused by material deposition or precipitation (usually solids, salts or hy- Solids, liquids and surfactant contaminants Flash tank Lean amine Rich amine Liquid hydrocarbon carry-under Hydrocarbon High energy usage Reboiler corrosion Rich amine suspended solids Liquid hydrocarbon contaminants FIG. 1. Amine unit diagram with common process difficulties. Hydrocarbon Processing | JUNE 2018 57 Process Optimization Corrosion. In amine units, corrosion is continuously caused during the removal of H2S or CO2 if carbon steel is present in the circuit. In some cases, corrosion can occur if the unit contains stainless steel. Passivation of metal surfaces by H2S is key for amine units; this is one reason why amine units only used for CO2 removal experience corrosion problems more often, as passivation of metal surfaces does not take place. Alternatively, deposition also generates corrosion. Typically, where there is accumulation of solids on surfaces, the natural progression is to find “under-deposit corrosion.” This is one of the most common corrosion mechanisms, and is caused by localized high concentrations of chemical species, ions and corrosion initiators. Typically, if the deposition of suspended solids is minimized, then corrosion rates are also reduced. One example of deposition is the consequence of increased corrosion rates in the regenerator of the amine unit. If the inlet temperature of the rich amine to the regenerator is lower than recommended (caused by deposition on the rich/lean amine heat exchanger, or by a temporary upset at the rich amine stream), then regeneration takes place at the bottom of the tower or reboiler. This type of corrosion mechanism is frequently encountered. FIG. 3 illustrates corrosion in stainless steel tubes in an amine unit reboiler. Heat-stable salts and amine decomposition products. Thermally stable salts are components that do not decompose or revert thermally in accordance with the normal conditions of the amine regeneration unit; they are called “heat-stable salts.” These salts typically require external treatments (such as ion exchange, electrodialysis or vacuum distillation) to remove them from the amine unit. Evidence exists that many of these salts cause accelerated rates of corrosion. Moreover, the surfaces of suspended solids have high molecular activity, as well as being rich in metals, such as iron. These solids may catalyze chemical reactions that form stable salts, further increasing their concentration. They can also cause the decomposition of the amine itself, possibly creating capacity limitations. Therefore, the reduction of suspended solids in the amine solution can mitigate (but not eliminate) the rate of forming stable salts and prolong the life of the amine solution. In general, heat-stable salts are a more common problem in amine units in refineries. In gas processing plants, they are somewhat less common. Foaming and emulsification. Foam is usually produced when gas contacts liquids with low surface tension. Foams are stabilized by surfactants or surfactant-like materials. Variation of surface tension by the addition of solvents, such as methanol, hydrocarbons or BTEX (benzene, toluene, ethylbenzene and xylene), may also contribute to foaming tendency and stability when surfactants are present. Solid surfactants may be smaller than 10 microns (iron sulfide), and they may also exist as liquid molecules (e.g., components in compressor lubricating oil or certain additives). The removal of surfactants and hydrocarbons (including BTEX) from the solvent will reduce the formation of foam and the need for antifoam additives. Excessive frothing with foaming invariably leads to loss of amine and reduced reaction efficiency with H2S. In some cases, the absorption of CO2 under foaming conditions is greater, which is undesirable for selective H2S removal. FIG. 4 shows amine solvent samples with high foaming tendency and stability. Emulsification is a different phenomenon that is somewhat related to foaming. It takes place when two liquid phases are mixed in the presence of a stabilizing surfactant. Amine units that process liquid hydrocarbons, such as liquified petroleum gas (LPG), condensates or natural gas liquids (NGL), often encounter emulsification events in the amine contactor. The main reason for emulsion formation is surface-active contaminants present in the feed liquid hydrocarbon. In some cases, however, the lean amine entering the liquid contactor FIG. 2. Deposits by suspended solids in column pall ring packing. FIG. 3. Corrosion in amine unit reboiler tubes. 58 JUNE 2018 | HydrocarbonProcessing.com FIG. 4. Amine solvent sample with strong foaming tendency. Process Optimization (commonly referred to as a liquid treater) can be a root cause of emulsification if amine degradation products are present. Emulsification may cause amine carryover into the treated hydrocarbon outlet of the amine unit. It can reach downstream units, such as caustic units and mercaptans removal units. This phenomenon can take place in either amine-continuous or hydrocarbon-continuous contactors. Filtration in amine units. In general, filtration and separation schemes in amine units are divided into three main areas: 1. Systems designed for the feed streams—e.g., gas streams, such as natural gas and vent gas, or liquid hydrocarbons, such as LPG and NGL 2. Systems for the circulating amine solution (lean and rich amine) 3. Systems to remove contaminants from the treated stream upstream of the next unit, or from the acid gas prior to final disposal or processing, or from the acid gases. It is important to also mention that lean and rich amine filtration and separation processes serve different purposes and protect different parts of the amine unit. Treated streams egressing from the amine unit contactor can impact downstream fuel gas systems in refineries and dehydration units in gas processing plants. In addition, acid gases from the amine unit regenerator can carry contaminants, such as hydrocarbons or amine solvent, that not only can affect downstream units, such as the sulfur recovery unit (SRU) process and the catalyst, in addition to burning or incineration, and acid gas reinjection into the ground, among others. The main filtration and separation areas indicated previously have different goals, functions and technologies. Therefore, they must be implemented correctly for the intended function. Each of the three filtration and separation methods (filtration, coalescing and adsorption) should operate in combination and synchronicity to enable effective performance of the amine unit, ensuring favorable, stable results in the process of removing H2S and CO2. Filtration of the full flow of the lean amine stream is always recommended. Particularly in cases where the contactor tower has packing, as opposed to trays, it is almost mandatory to have a full flow amine filter. Filtration may minimize solids deposits and fouling of the tower internals. After the full flow lean amine filtration, a percentage (typically 20%–25% minimum) of the flow should be routed to the activated carbon bed. A post-filter sized according to the flow entering the activated carbon bed should also be used. It is best to avoid high-temperature amine filtration, such as filtration systems placed directly at the outlet of the regenerator. High-temperature filtration results in limited FIG. 5. Corroded and collapsed central metal core of a cartridge filter that was installed in a lean amine stream directly out of a regenerator atPumpAd_3_5x4_625_f.qxp_Layout 121°C (250°F). 1 10/24/17 3:04 PM Page 1 Thousands of Pumps Have Never Seized Lean amine solvent filtration. Proper purification of the lean amine stream is accomplished by using two filtration stages and one adsorption stage. Lean solvent mechanical filtration is designed to protect the activated carbon bed, as well as the amine unit contactor. Lean amine filtration is complementary to adsorption, which is intended for soluble contaminant removal. Lean amine filtration and adsorption are normally composed of three different stages. Each stage has a specific function. All steps are necessary and cannot be replaced or eliminated. The core of the process is the activated carbon bed. The activated carbon bed is not truly a “filter,” as it is often called. The proper definition of filtration is the separation of solid particles from a liquid using a porous or fibrous medium. Activated carbon beds should not act as filters and should function only as a physical adsorption bed for dissolved contaminants. Contaminants are adsorbed with weak interactions within the surface pores of the activated carbon grains. Therefore, it is necessary to keep the activated carbon surface free of solids. For this to happen, the activated carbon beds must always be protected with an effective pre-filter; otherwise, particulates will collect in the bed and cause high differential pressure. This could necessitate backflushing of the bed, or other removal of those particulates. Pumps fitted with GRAPHALLOY® wear parts survive upsets. • Run dry and keep running • Self-lubricating • Non-galling • Won’t swell • Corrosion resistant • Dimensionally stable • Improved efficiencies • -400˚F to 1000˚F (-240˚C to 535˚C) +1.914.968.8400 Yonkers, NY USA • GRAPHITE METALLIZING CORPORATION Yonkers, NY USA P1 www.GRAPHALLOY.com Select 161 at www.HydrocarbonProcessing.com/RS 59 Process Optimization thermal and chemical compatibility of materials for many filters. When using filtration out of the regenerator, careful materials screening is necessary. Media such as flame-resistant polymers and cellulose can be used, in addition to metal media. Lowertemperature filtration allows for additional media materials, such as polypropylene and epoxy-coated fiberglass, to be used. At present, the best filtering devices for amine units are disposable cartridge filters, which are also the most effective for filtration of deformable particles, especially when hydrocarbons are present. Automatic (back-washable) filters with metal media materials are unsuitable for amine units because contaminants tend to have strong adhesion to metal surfaces. The strong interactions of metal filter elements with suspended solids often render backwash or automatic filter cleaning ineffective. Other devices, such as centrifuges, have been used with success in certain facilities, although maintenance of rotating equipment must be considered. Technical hurdles, such as flow and pressure limitations, as well as reliability, must be overcome for this technology to be used in amine plants. Filters with filter aids and precoatings have also been used, but these are less common due to difficult waste disposal, complex maintenance procedures and operational issues. However, some of these devices have been used in large amine units with large amine solvent flows. Regarding cartridge filtration, numerous media materials are used in amine service. Examples of the most common materials are cotton, polymer-impregnated cellulose, polypropylene and polymer-impregnated fiberglass. Care must be taken to use only filter media that are compatible with the amine solvent and do not cause foam formation by reacting with the material or extraction of components. Foam caused by filters may originate due to the material used in the filter (epoxy adhesives, filter media, release agents used during manufacture or the endcap material), or by incompatibilities of contaminants in the amine solvent with the filter (e.g., BTEX). Filter media with polypropylene fibers should be used carefully because of the low softening-point temperature. Hightemperature excursions could cause the filter materials to melt. Also, filters with polyester materials should not be used in amine units because they undergo basic hydrolysis or aminolysis reactions with the amine solvent, degrading and dissolving the material. In both cases, thermal and chemical incompatibility of the filter material can lead to foam formation or contamination of equipment surfaces, requiring total cleaning of the unit. Finally, it is important to mention that when cellulose filter media are used, tests should be conducted to ensure that foam TABLE 1. Different amine unit filter types and key properties Filter type/ details Cost Loading capacity Flow Bypass Bag Low Low/medium In-out Melt-blown Low/medium Low Out-in More often Resin-bonded Low/medium Low Out-in More often More often String-wound Low Low Out-in More often High-flow High High In-out Less often Pleated cartridge Medium/high High Out-in Less often Notes: S = surface filtration, D = depth filtration 60 JUNE 2018 | HydrocarbonProcessing.com formation is not a consequence of the cellulose material. Some cellulose filter media materials are actually incompatible with amine solvents and should not be used. It is advised to always test both chemical and thermal compatibilities with the amine solvent before any filter is used. This is especially true for the lean amine circuit directly out of the regenerator, where high temperatures are prevalent. FIG. 5 shows the central metal core of a cartridge filter installed in a lean amine side, directly out of the unit regenerator. The high temperatures 121°C (250°F), coupled with the aggressive conditions of a formulated amine solvent, corroded and weakened the filter metal core to the point of collapse. The degree of filtration and efficiency is discussed in more detail in the next section. However, any initial filtration efficiency and media pore size or micron rating selection is only a starting point. Filtration optimization and efficiency selection for the amine solvent is best when the amine unit is operational and online gravimetric analysis of a slipstream is conducted. Online gravimetric analysis at the inlet and outlet of a filter will accurately determine the efficiency of the filter media used in the system; only afterward is it possible to adjust the efficiency and achieve best results, acceptable filtration costs and proper filtration levels. From a filter design standpoint, it is necessary to ensure a maximum flux inflow of 0.5 gal/min/ft2 of filter media surface area. However, lower fluxes will produce not only better filtration, but they will also reduce operational costs, operator exposure and waste generation. The drawbacks of low-flux filtration are higher capital expenses (marginal compared to the cost of an amine unit) and larger system footprints. Lean amine solutions with high solids content are less likely to produce efficient stripping of H2S and CO2. This inefficiency is caused by the formation of a multi-layer solid interface between the acid gases (or liquids) and the amine solvent, essentially reducing mass transfer. A high concentration of suspended solids in amine solvents is usually an indication of a detrimental situation, such as high corrosion, taking place in the unit. Such issues should be investigated promptly. In general, amine units with high-quality amine solutions have minimal suspended solids (1 ppmw or less), resulting in better performance and a more stable process. In essence, filters avoid equipment damage and protect the unit internals and amine solvent. Filters also reduce unit maintenance costs and energy use. Whereas cartridge filtration is a good avenue for amine filtration, it fails to perform when “shoe-polish” material is present in the amine unit. This shoe-polish material is usually more common in refineries and gas processing plants with amine solvents with high heat-stable salts and iron sulfides. It is similar to a gel material, has a black color and is usually fairly soluble in water. Common Type The material is a mixture of iron sulfide, sizes, in. heat-stable salts, traces of hydrocarbons 6 × 30 S and polymerized amine, and requires a much more careful and rigorous filtration. 2.5 × 40 D The amorphous material contains solid 2.5 × 40 D particulate iron sulfides, predominantly 2.5 × 40 D between 1 microns and 5 microns in size. 6 × 60 S This material also causes a rapid increase in filter pressure differential, thereby leading 2.5 to 6.5 S OD × 40 to a rapid decrease in filter lifetime. The formation of shoe-polish material Process Optimization should be monitored and prevented by maintaining low suspended solids and corrosion rates, ensuring that amine degradation is minimized and heat-stable salts are kept to low levels, and not using silicone antifoams, if possible. Silicone antifoam products are good for removing foam from liquids, but they often negatively affect the amine process through solids or semi-solids deposition. In terms of amine solvent filters, disposable filter elements (internals) are the most common for both lean amine and rich amine circuits. Some examples of disposable filters are shown in FIG. 6. Common filters used in amine units include bag filters, pleated cartridge filters, depth-style cylindrical filters and string-wound filters. All filter element designs have a number of pros and cons, and should be properly evaluated for use in amine units. It is necessary to use proper sealing mechanisms to ensure that no bypass takes place inside the vessel. Filters normally use flat gaskets, as shown in FIG. 6 (right); however, O-rings are much better because they offer an efficient sealing method. Flat gaskets often must be affixed to the filter using an adhesive. Several adhesives have been observed to fail if chemically incompatible with amine solvents. Gaskets and O-rings used in amine units should be ethylene-propylene-diene-monomer (EPDM) rubber, as they offer good compatibility and stability for the process. Other types of filters, such as automatic systems, have not shown successful results in amine units due to their ineffective cleaning results. Solids in amine units tend to FIG. 6. Common disposable filters used in amine units. Left to right: cotton-wound, polypropylene melt-blown and pleated styles. FIG. 7. Amine solvent samples. Left: new amine solvent; right: contaminated amine solvent. 62 JUNE 2018 | HydrocarbonProcessing.com be highly fouling and strongly adherent to metal surfaces, hindering backwash or mechanical removal by automatic filers. Finally, some amine units have used centrifuges with good results; however, reliability and the inability to tolerate high pressures make them less common in the industry today. All filter elements will filter suspended solids to some extent when installed in an amine unit. Some are more effective or have lower costs than others. Field experience has shown that the pleated cartridge filters, operating as surface filters where solids are accumulated at the filter surface, often perform better compared to the other types. In addition, the cost performance is the best among other filters. Bag filters are low-cost, but they do not have the efficiency or the surface area for proper amine solvent filtration. Cotton string-wound filters display good chemical compatibility with the amine solvent, but they lack contaminant removal capacity, and efficiency decreases as the media deforms from high differential pressure. Depth filters, where the suspended solids are immobilized in the inner structure of the filter element, are also less effective because solids in amine units are usually filtered at the surface of the filter media and form a cake or seal off the surface. This eliminates the benefits of depth filtration, leading to short online life. TABLE 1 summarizes various amine unit filter types and key properties. Lean amine solvent activated carbon beds. Activated carbon can be manufactured from various materials, such as wood, coal, lignite, coconut shell, peat or other organic sources. Activated carbon can be in powder form, granular or extruded in various shapes. The purpose of activated carbon is to remove dissolved organic components by adsorption. These dissolved organics often generate foam in amine unit solvents, so their removal is beneficial to the operation of the amine unit. Other activated carbon functions include odor control and color improvement. Dissolved contaminants, such as surfactants and chemical additives, that lead to foam are generally dissolved hydrocarbons or surfactants that interact with the activated carbon surface due to the pore structure of activated carbon. As adsorbent media, activated carbon removes, on average, 5%–10% of its weight in organic species, if operating below the material’s isotherm. Depending on the type of contaminants and operating conditions (temperature, flow velocity, activated carbon type, pore size and activation mode), the capacity of the carbon may vary. Activated carbon beds are best employed in lean amine streams after the heat exchangers and coolers. Lower temperatures lead to better adsorption. The use of activated carbon beds in the rich amine stream is not recommended because of difficult maintenance procedures, operator exposure and waste generation/disposal. Spent activated carbon in rich amine streams may contain high concentrations of H2S, thereby requiring additional steps for changeout. Activated carbon used in liquid streams is usually granular or extruded as pellets, and not in powder form given the higher drag, increased entrained fines and higher differential bed pressure. However, the efficiency of granular activated carbon is higher compared to the pelletized form. All types of activated carbon suffer structural fractures and generate solids that are carried out with the outgoing process stream (carbon fines). Granular carbon—and, to a lesser degree, extruded/pellet- Process Optimization ized carbon—often fracture during installation and operation, making it necessary to backwash the material to remove as many fines as possible, as well as to separate any carbon fines at the outlet of the carbon bed before they can enter the amine unit contactor. Some activated carbons are also activated with certain acids, and the backwash ensures that acid residues are removed. High suspended solids entering the amine unit contactor will cause solids deposition, possible erosion/corrosion and, in some cases, foaming due to foam stabilization by suspended solids. These scenarios can cause lower H2S removal efficiency in the amine unit, equipment failures and other problems. In many cases, carbon fines from the lean amine can reach all the way to the rich amine circuit, causing deposits in the flash tank and reducing residence time. Deposits may also be found in the heat exchanger, reducing rich amine temperature to the amine unit regenerator; and the regenerator tower, possibly leading to erosion/corrosion at the bottom of the regenerator. For removing solids at the outlet of the activated carbon bed, a general guideline of 5-micron (beta5 5000, 99.98% efficiency) filters are required, using surface filtration. However, this is only a starting point, and the filter micron ratings should be adjusted FIG. 8. Various activated carbon types, origins and associated pore structures. Image courtesy of Calgon. FIG. 9. Lean amine filtration system (pre-filter and post-filter) with activated carbon bed. 64 JUNE 2018 | HydrocarbonProcessing.com based on the particle size distribution of the amine solvent leaving the carbon bed. Note: The beta5 5000 filter efficiency is the ratio of the number of particles exiting the filter and the number of particles entering the filter, for particles 5 microns and larger (i.e., the reason for the subscript 5 notation). Activated carbon that is activated with and/or rinsed with phosphoric acid or phosphorous-based additives should not be used in amine units because of the potential for foaming. Only heat- or steam-activated carbon should be used. A properly designed activated carbon bed can considerably reduce foaming from amine solvents by removing surfactants, dissolved hydrocarbons and amine degradation products. Activated carbon beds are generally sized for a 20%–25% minimum slipstream flow. It is best to have at least 25%–50% slipstream flow of the total circulating amine solution. A minimum contact time of 15 min. and a cross-sectional flow velocity of 2 gal/min/ft2–3 gal/min/ft2 are considered suitable for a correctly designed activated carbon bed. Finally, the design of the inlet distributor is key to carbon beds, as it ensures the most efficient use of the entire bed. When the amine solution changes color or presents haziness, or when foaming tendency is increased, the carbon bed must be evaluated and replaced, if necessary. Color changes are associated with dissolved metals and amine degradation products. Foam is generally caused by surfactants, haziness, suspended solids, dissolved metals from corrosion or hydrocarbon emulsions. FIG. 7 shows amine samples in a gas processing plant. The bottle on the left represents a new amine solvent, and the bottle on the right is a contaminated amine solvent. The contaminated amine solvent sample presents considerable haziness from emulsified hydrocarbon and oil, in addition to a separate upper-hydrocarbon phase. Activated carbon beds should not be changed out solely due to pressure drop, since this parameter is a misrepresentation of bed efficiency. In fact, activated carbon beds should not experience any increase in differential pressure. When differential pressure increases in a carbon bed, it is necessary to replace the bed because of solids or hydrocarbon obstruction of the carbon grain pores, which hinders contaminants removal. To avoid solids buildup inside the carbon bed, it should be protected by a pre-filter. The pre-filter micron size for the protection of carbon beds is often 10 microns (beta10 5000 or 99.98% efficiency) and surface filtration. However, this is only a starting point, and the filter micron ratings and micron size should be adjusted based on the particle size distribution of the amine solvent entering the bed, ideally at the time of processing into the carbon bed. Activated carbon bed lifetimes are typically 6 mos–12 mos. This industry guideline is not always consistent, however, because carbon bed lifetimes depend on several factors, such as bed size, bed design, internal flow velocity, contaminant concentration and carbon type. Therefore, evaluating carbon beds for replacement is complex. The best way to evaluate bed lifetime is to take samples of the carbon inside the bed and test for its activity (molasses number, methylene blue number and iodine number). However, sampling is not straightforward, as it depends largely on where and how the sample of carbon was taken inside the bed. In addition, carbon-sampling ports are not an existing feature in most carbon beds. At present, the only way to test for a spent carbon bed and determine its life is by using a surface rheology (and sometimes Process Optimization surface tension) analysis of the amine solvent at the inlet and outlet of the bed, and then comparing the results. A simpler test is to compare the foaming tendency of amine into and out of the bed. Also, the outlet amine solvent should be lighter in color and lower in viscosity. The origin of activated carbon generally defines its adsorption power, capacity and pore size distribution. A general guideline for activated carbon use in amine units is as follows: If the amine unit has an efficient feed gas coalescer, then the type of activated carbon used should be granular, 8 × 30 (mesh), of bituminous origin and activated by steam. This type of activated carbon has the most suitable balance of pore sizes (small, medium and large) capable of adsorbing a wide range of contaminants in the amine solution. However, if the amine unit does not have a feed gas coalescer or has an inefficient feed gas coalescer, then the most suitable activated carbon type is granular, 4 × 10 (mesh), from lignite. This type of activated carbon has a greater balance of larger carbon pores, allowing for better absorption of larger molecules, such as hydrocarbons or surfactants, that cause foaming. FIG. 8 shows a diagram of the various activated carbon types based on origin and overall pore size distribution. It can be seen that the pore size distribution is very different depending on the carbon type. The rationale for using lignite carbon when poor inlet separation is present is to have larger pore sizes to accommodate larger surfactant molecules. Special types of activated carbons are also available. They generally come from the family of impregnated activated carbons that contain chemical additives to perform certain specific functions, such as sulfur-impregnated activated carbon for the removal of vapor-phase mercury in feed gas to processing plants. Activated carbon beds are recommended for bypass mode in only three scenarios: • When combating foaming events with antifoams. Antifoams will be removed in the carbon bed to a certain level, depending on the antifoam product. The bed will exhaust much more rapidly, and the antifoam will be rendered ineffective. • During plant startup due to high-solids loads in the system. • During ingress of liquid hydrocarbons causing amine emulsification. Activated carbon beds should always be a three-stage process that includes pre-filtration, carbon bed adsorption and post-bed filtration. FIG. 9 shows a refinery lean amine filtration system with an activated carbon bed in the center. The bed is flanked by duplex pre-filters to the left and the duplex post filters to the right. Duplex (or parallel) filters are commonly used when a constant flow through a filter is desired; they work by alternating between the two filters when maintenance is performed. This arrangement represents a correct activated carbon bed system configuration. Guidelines for lean amine filtration and adsorption. In amine units, the lean amine stream requires both filtration and adsorption purification processes. The stream often contains suspended and dissolved contaminants that must be removed. Below is a general set of filtration and adsorption guidelines for amine units. FIG. 10 shows a basic flow diagram of lean amine flow from the heat exchanger to the absorber. Recommended activated carbon system parameters in amine units include: • If an efficient inlet gas coalescer is in place at the unit, then the unit should use bituminous activated carbon, granular, 8 × 30 mesh size. • If an inefficient, undersized or no inlet gas coalescer is in place, then the unit should use lignite activated carbon, granular, 4 × 10 mesh size. (Note: Grain size is irrelevant here). • Steam- or heat-activated. If acid-activated, avoid phosphoric acid. When using acid-activated carbon, the materials must be fully rinsed by reverse flow to a neutral pH. • Process 25%–50% of lean amine flow for best results. • Activated carbon pre-filter. To appropriately select the level of pre-filtration efficiency, it is necessary to analyze the lean amine particle size distribution and total suspended solids. However, if this is unavailable, a good starting point is 10 microns (beta10 5000/99.98% efficiency). Lean filter flow is normally 25% Recommended to split flow after precarbon filter Lean amine flow Lean amine Post-carbon Pre-carbon Carbon filter filter bed From lean/rich exchanger Amine cooler Booster pump FIG. 10. Typical lean amine filtration flow diagram. Only One Foot Required. Roth Low NPSH pumps require a Net Postive Suction Head (NPSH) of only one foot of liquid for full curve performance. Roth chemical processing pumps include a standard chemical duty, low NPSH, seal less magnetic drive, and low NPSH multistage pump options to pump an extensive array of chemicals including liquefied gases. 1-888-444-ROTH • www.rothpump.com Select 162 at www.HydrocarbonProcessing.com/RS 65 Process Optimization • Activated carbon post-filter. To appropriately select the level of post-filtration efficiency, it is necessary to analyze the lean amine particle size distribution and total suspended solids. However, if this is unavailable, a good starting point is 5 microns (beta5 5000/99.98% efficiency). The split flow to the carbon bed is commonly placed upstream of the pre-filter with a 75% bypass and only 25% of the flow processed into the carbon bed. This leaves the contactor predominately unprotected. For this reason, it is recommended to place the split flow after the pre-filter to provide full-flow prefiltration and to better protect the contactor from suspended solids. Finally, it is necessary to emphasize that activated carbon beds should be installed in the cool lean amine side, just before the unit contactor. The objective is to properly clean the amine solvent of any soluble contaminant that can cause foaming prior to entering the contactor. To achieve this objective, operating the activated carbon bed at lower temperatures favors adsorption, making it more effective. Using rich amine-activated carbon beds or operating the activated carbon process at high temperatures is not recommended. These cases are often plagued with problems, low effectiveness and negative results. Takeaway. Contamination control is a critical step in any optimization process—especially in an amine unit, where contamination is often strongly linked with process performance. Lean amine filtration and adsorption play a critical role in amine Liquefied Natural Gas REAL-TIME LNG DATA Any project. Any time. Any place. Updated in real time, the web-based platform enables users to access worldwide facility and project data. Actionable Data • Owner/Operator • Project status • Project storage and capacity • Shareholder data • LNG shipping/vessel information • Global Shipping Ports • Global Natural Gas Pipelines • Process method 66JUNE 2018 | HydrocarbonProcessing.com Discover more at EnergyWebAtlas.com units. The fundamental parameters and lessons shared in Part 1 of this article can be applied to most, if not all amine units. It is important to differentiate lean amine filtration from rich amine filtration, as both have different purposes and objectives. Lean amine filtration and adsorption protects the amine unit contactor (or absorber) and, in certain cases, the lean-rich heat exchanger, especially for fouling and foam minimization. The rich amine filtration (and sometimes coalescence) protects the leanrich heat exchanger and, in particular, the regenerator. In addition, the rich amine filtration ensures that any downstream units that process the acid gas are also protected. In Part 2 of this article, fundamental concepts for rich amine filtration and inlet separation will be discussed. DAVID ENGEL is Managing Director of Nexo Solutions and Global Technology Leader for Exion Systems. He holds a BS degree in industrial chemistry from the University of Santiago, Chile, and a PhD in organic chemistry from Indiana University in Bloomington, Indiana. Dr. Engel has published more than 75 articles and has 18 invention patents in his name. P. SCOTT NORTHROP is a gas treating advisor in the facilities function of ExxonMobil’s Upstream Research Co. in Houston, Texas. He received his BS degree from Washington University in St. Louis, Missouri, and an MS degree and PhD from the California Institute of Technology, all in chemical engineering. Dr. Northrop has 28 yr of experience in the industry, and is the author/co-author of a number of patents, presentations and articles in a variety of related subjects. He sits on Technical Section F of the Gas Processors Association (GPA), and on the board of directors of Alberta Sulfur Research Ltd., Canada.