Uploaded by
Abdalrahman Aboelmaaty
Aldehydes and Ketones: Structure, Properties, Reactions
advertisement

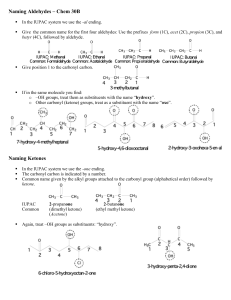
Aldehydes and Ketones Introduction Aldehydes are compounds of the general formula RCHO; Ketones are compounds of the general formula RR´CO. The groups R and R´ may be aliphatic or aromatic. O O O C C C R H Aldehyde R R Ketone Carbonyl group Both aldehydes and ketones contain the carbonyl group, C=O, and are often called carbonyl compounds. An aldehyde is often written as RCHO. Remember that the H atom is bonded to the carbon atom, not the oxygen. Likewise, a ketone is written as RCOR, or if both alkyl groups are the same, R2CO. Each structure must contain a C––O for every atom to have an octet. The three bonds (carbon, oxygen, and the two other atoms attached to carbonyl carbon) lie in a plane; the three bond angels of carbon are very close to 120º. Nomenclature Both IUPAC and common names are used for aldehydes and ketones. Naming Aldehydes in the IUPAC System To name an aldehyde using the IUPAC system: [1] If the CHO is bonded to a chain of carbons, find the longest chain containing the CHO group, and change the -e ending of the parent alkane to the suffix -al. If the CHO group is bonded to a ring, name the ring and add the suffix -carbaldehyde. [2] Number the chain or ring to put the CHO group at C1 Example: Give the IUPAC name for the compound: Give the IUPAC name for the compound: H H O2N C O p-nitrobenze carbaldehyde H3C C O p-methylbenze carbaldehyde Common Names for Aldehydes The common names of aldehydes are derived from the names of the corresponding carboxylic acids by replacing –ic acid by –aldehyde. Greek letters are used to designate the location of substituents in common names. The carbon adjacent to the CHO group is the ` carbon, and so forth down the chain. Naming Ketones in the IUPAC System To name an acyclic ketone using IUPAC rules: [1] Find the longest chain containing the carbonyl group, and change the -e ending of the parent alkane to the suffix -one. [2] Number the carbon chain to give the carbonyl carbon the lower number. Apply all of the other usual rules of nomenclature. Common Names for Ketones Most common names for ketones are formed by naming both alkyl groups on the carbonyl carbon, arranging them alphabetically, and adding the word ketone. Using this method, the common name for 2-butanone becomes ethyl methyl ketone. O O O H3C C CH3 H3CH2C C CH3 H3CH2CH2C C CH3 Propanone Acetone Butanone Methyl ethyl ketone 2-Pentanone O O C CH3 C CH2CH2CH3 Acetophenone n-Butyrophenone O C Benzophenone Physical properties: Boiling point: Aldehydes and ketones are polar compounds due to the polarity of carbonyl group and hence they have higher boiling points than non polar compounds of comparable molecular weight. But they have lower boiling points than comparable alcohols or carboxylic acids due to the intermolecular hydrogen bonding. Solubility: The lower aldehydes and ketones soluble in water, because of hydrogen bonding between carbonyl group and water, also they soluble in organic solvents. Preparation of aldehydes & Ketones. Preparation of aldehydes. 1. Oxidation of primary alcohols: Primary alcohols can be oxidized to give aldehydes by using of K2Cr2O7. RCH2OH + Cr2O7-1o alcohol Orange-red H R C O + Cr+++ An aldehyde K2Cr2O7 OH R C O A carboxylic acid Green O 2- synthesis of benzaldehyde CHCl2 C H H2O CH3 Cl 2 t a e ,h O ace t CrO ic a 3 nh yd rid e O CH(OCCH3)2 C H H2O 3. Partial reduction of acid chlorides Strong reducing agents (like LiAlH4) reduce acid chlorides all the way to primary alcohols. Lithium aluminum tri(t-butoxy)hydride is a milder reducing agent that reacts faster with acid chlorides than with aldehydes. Reduction of acid chlorides with lithium aluminum tri(t-butoxy)hydride gives good yields of aldehydes. (-78ºC) Preparation of Ketones 1. Oxidation of Secondary alcohols: Secondary alcohols are oxidized to ketones by chromic acid (H2CrO4) in a form selected for the job at hand: aqueous K2Cr2O7, CrO3 in glacial acetic acid, CrO3 in pyridine, etc. Hot permanganate also oxidizes alcohols; it is seldom used for the synthesis of ketones. R- RR C OH H A 2o alcohol K2Cr2O7 or CrO3 R C O A ketone 2. Cleavage of Carbon–Carbon double bond by Ozone: Oxidative cleavage of an alkene breaks both the σ and π bonds of the double bond to form two carbonyl groups. Depending on the number of R groups bonded to the double bond, oxidative cleavage yields either ketones or aldehydes. 3. Friedel-Crafts acylation. The Friedel-Crafts reaction involves the use of acid chlorides rather than alkyl halides. An acyl group (RCO–) becomes attached to the aromatic ring. Thus forming a ketone; the process is called acylation. O Ar H + R C Cl O AlCl3 or other Lewis acid Ar C R + HCl 4. Hydration of alkynes. Alkynes undergo acid-catalyzed addition of water across the triple bond in the presence of mercuric ion as a catalyst. A mixture of mercuric sulfate in aqueous sulfuric acid is commonly used as the reagent. Reactions a) Addition of Alcohols (Acetal Formation): Aldehydes and ketones react with two equivalents of alcohol to form acetals. In an acetal, the carbonyl carbon from the aldehyde or ketone is now singly bonded to two OR" (alkoxy) groups. b) Nucleophilic Addition of CN– : Treatment of an aldehyde or ketone with NaCN and a strong acid such as HCl adds the elements of HCN across the carbon–oxygen π bond, forming a cyanohydrin. H C O NaCN NaHSO3 Benzaldehyde H C CN OH Mandelonitrile O H3C C CH3 CH3 CH3 + NaCN Acetone H2SO4 H3C C CN H2O, H2SO4 H3C COOH C OH OH Acetone cyanohydrin - H2O CH3 H2C C COOH Methacrylic acid c) Addition of organometallic reagents (R–) The addition of Grignard reagents to aldehydes and ketones yields alcohols. The organic group, transferred with a pair of electrons from magnesium to carbonyl carbon, is a powerful nucleophile. O C + R: MgX C OMgX R H2O C OH R + Mg(OH)X H+ Mg++ + X- + H2O d) Addition of derivatives of Ammonia (Formation of imine). Treatment of an aldehyde or ketone with a 1° amine affords an imine (also called a Schiff base). Nucleophilic attack of the 1° amine on the carbonyl group forms an unstable carbinolamine, which loses water to form an imine. The overall reaction results in replacement of C=O by C=NR. Oxidation reaction Aldehydes are readily oxidized to yield carboxylic acids; but ketones are generally inert toward oxidation. The difference is a consequence of structure: aldehydes have a –CHO proton that can be abstracted during oxidation, but ketones do not. Hydrogen here O C R H An aldehyde [O] R O O C C OH Carboxylic acid R R A ketone No hydrogen here Many oxidizing agents, including KMnO4 and hot HNO3, convert aldehydes into carboxylic acid. But CrO in aqueous acid is a more common choice in the laboratory. The oxidation occurs rapidly at room temperature and results in good yields. 3 hot HNO3 RCHO or ArCHO KMnO4 K2Cr2O7 RCOOH or ArCOOH Methyl ketones: Oxidation of ketones required breaking of C–C bond next to the carbonyl group and takes place only under vigorous conditions, except for methyl ketones which oxidized smoothly by mean of hypohalite (NaOX) to form Haloform (Haloform reaction). Aldol condensation Under the influence of dilute base or dilute acid , two molecules of an aldehyde or a ketone, which contained α-hydrogen , may combine to form a β-Hydroxy aldehyde or β-Hydroxy ketone. This reaction is called the Aldol condensation . Aldehyde alcohol Mechanism: If aldehyde or ketone does not contain an α-hydrogen, a simple Aldol condensation cannot take place. For example: ArCHO HCHO (CH3)3CCHO ArCOAr ArCOCR3 No -hydrogen atoms dilute OH- No reaction Cannizzaro reaction. In the presence of concentrated alkali, aldehydes containing no α-hydrogen undergo self-oxidation and reduction to yield a mixture of an alcohol and a salt of a carboxylic acid. This reaction is known as the Cannizzaro reaction. H 2 C O An aldehyde with no hydrogen strong base COOAcid salt + CH2OH Alcohol Examples: H 50% NaOH 2 H C O Formaldehyde 2 O2N CHO room temp. 35% NaOH p-Nitrobenzaldehyde O2N H COO+ Formate ion CH2OH 50% KOH + 2 m-Chlorobenzaldehyde COO- Na+ Sodium p-nitrobenzoate COO- Cl Methanol CH2OH + O2N p-Nitrophenyl alcohol CHO CH3OH Cl m-Chlorobenzoate ion Cl m-Chlorobenzyl alcohol