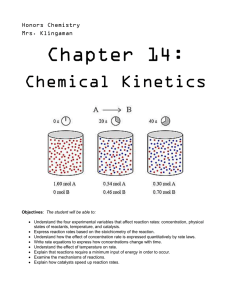
AP CHEMISTRY Test Booklet 5.11 Catalysis 1. The energy diagram for the reaction X + Y → Z is shown above. The addition of a catalyst to this reaction would cause a change in which of the indicated energy differences? (A) I only (B) II only (C) III only (D) I and II only (E) 2. I, II, and III Answer the following questions in terms of thermodynamic principles and concepts of kinetic molecular theory. (a) Consider the reaction represented below, which is spontaneous at 298 K. CO2(g) + 2 NH3(g) → CO(NH2)2 (s) + H2O(l) ΔHo298 = -134kJ (i) For the reaction, indicate whether the standard entropy change, ΔSo298 is positive, or negative, or zero. Justify your answer. (ii) Which factor, the change in enthalpy, ΔHo298 or the change in entropy, ΔSo298, provides the principal driving force for the reaction at 298 K? Explain. (iii) For the reaction, how is the value of the standard free energy change, ΔGo, affected by an increase in temperature? Explain. (b) Some reactions that are predicted by their sign of ΔGo to be spontaneous at room temperature do not proceed at a measurable rate at room temperature. (i) Account for this apparent contradiction. (ii) A suitable catalyst increases the rate of such a reaction. What effect does the catalyst have on ΔGo for the reaction? Explain. Please respond on separate paper, following directions from your teacher. AP Chemistry Page 1 of 17 Test Booklet 5.11 Catalysis 3. For parts of the free-response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate. The hydrocarbon cyclobutane, decomposes into ethene, . , is represented above. At high temperatures, cyclobutane quickly (a) Draw a Lewis electron-dot diagram of the ethene molecule in the following box. Page 2 of 17 AP Chemistry Test Booklet 5.11 Catalysis Please respond on separate paper, following directions from your teacher. (b) Estimate the value of the bond angle in ethene. Please respond on separate paper, following directions from your teacher. (c) The graph below shows the concentration of cyclobutane as it decomposes over time at AP Chemistry . Page 3 of 17 Test Booklet 5.11 Catalysis (i) Based on the graph, determine the order of the decomposition reaction of cyclobutane at answer. . Justify your Please respond on separate paper, following directions from your teacher. (ii) Calculate the time, in milliseconds, that it would take for 99 percent of the original cyclobutane at decompose. to Please respond on separate paper, following directions from your teacher. Another alkane with a ring structure is cyclopentane, . Cyclopentane reacts with chlorine, , to and . Following is a representation of a proposed mechanism for the reaction. produce (slow) (fast) (fast) Please respond on separate paper, following directions from your teacher. (d) Write a rate law for the reaction that is consistent with the mechanism. Justify your answer. Please respond on separate paper, following directions from your teacher. (e) A student claims that is a catalyst in the reaction. Explain why the student’s claim is false. Please respond on separate paper, following directions from your teacher. Page 4 of 17 AP Chemistry Test Booklet 5.11 Catalysis 4. The energy distribution profile (Curve C) for the molecules is shown in the graph above for the reaction when it is done under certain experimental conditions. Line A represents the most probable molecules, and Line B represents the activation energy. Which of the following changes should be energy of the made to the graph to explain the increase in the rate of the reaction if the only change in experimental conditions is the addition of a catalyst? (A) Curve C will broaden and Line B will move to the left, because more molecules will have an energy greater than the minimum energy needed to overcome the activation energy barrier. (B) Line B will move to the left because a larger fraction of the overcome the activation energy barrier. (C) Curve C and Line A will move to the right because the average energy of the molecules will have the minimum energy to (D) Line A will move to the right because the most probable energy of the molecules will increase. molecules will increase. AP Chemistry Page 5 of 17 Test Booklet 5.11 Catalysis 5. For parts of the free response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate. Methanoic acid, , ionizes according to the equation above. (a) Write the expression for the equilibrium constant, , for the reaction. Please respond on separate paper, following directions from your teacher. (b) Calculate the of a solution of . Please respond on separate paper, following directions from your teacher. (c) In the box below, complete the Lewis electron-dot diagram for valence electrons. . Show all bonding and nonbonding Please respond on separate paper, following directions from your teacher. Page 6 of 17 AP Chemistry Test Booklet 5.11 Catalysis (d) In aqueous solution, the compound is combined with a reacts according to the equation above. A sample of . (i) Write the balanced net ionic equation for the reaction that occurs when . sample of is combined with Please respond on separate paper, following directions from your teacher. (ii) Is the resulting solution acidic, basic, or neutral? Justify your answer. Please respond on separate paper, following directions from your teacher. When a catalyst is added to a solution of occurs. , the reaction represented by the following equation (e) Is the reaction a redox reaction? Justify your answer. Please respond on separate paper, following directions from your teacher. AP Chemistry Page 7 of 17 Test Booklet 5.11 Catalysis (f) The reaction occurs in a rigid vessel at , and the total pressure is monitored, as shown in the graph above. The vessel originally did not contain any gas. Calculate the number of moles of produced in the dissolved in the solution is negligible.) reaction. (Assume that the amount of Please respond on separate paper, following directions from your teacher. (g) After the reaction has proceeded for several minutes, does the amount of catalyst increase, decrease, or remain the same? Justify your answer. Please respond on separate paper, following directions from your teacher. Page 8 of 17 AP Chemistry Test Booklet 5.11 Catalysis 6. Answer each of the following in terms of principles of molecular behavior and chemical concepts. a. The structures for glucose, C6H12O6 , and cyclohexane, C6H12 , are shown below. Identify the type(s) of intermolecular attractive forces in i. pure glucose ii. pure cyclohexane b. Glucose is soluble in water but cyclohexane is not soluble in water. Explain. c. Consider the two processes represented below. Process 1: H2O(l) → H2O(g) ∆H° = +44.0 kJ mol−1 Process 2: H2O(l) → H2(g) + ½ O2(g) ∆H° = +286 kJ mol−1 i. For each of the two processes, identify the type(s) of intermolecular or intramolecular attractive forces that must be overcome for the process to occur. ii. Indicate whether you agree or disagree with the statement in the box below. Support your answer with a short explanation. When water boils, H2O molecules break apart to form hydrogen molecules and oxygen molecules. d. Consider the four reaction-energy profile diagrams shown below. AP Chemistry Page 9 of 17 Test Booklet 5.11 Catalysis i. Identify the two diagrams that could represent a catalyzed and an uncatalyzed reaction pathway for the same reaction. Indicate which of the two diagrams represents the catalyzed reaction pathway for the reaction. ii. Indicate whether you agree or disagree with the statement in the box below. Support your answer with a short explanation. Adding a catalyst to a reaction mixture adds energy that causes the reaction to proceed more quickly. Please respond on separate paper, following directions from your teacher. Page 10 of 17 AP Chemistry Test Booklet 5.11 Catalysis 2 H2O2(aq) → 2 H2O(l) + O2(g) 7. The decomposition of hydrogen peroxide to form water and oxygen gas is represented by the equation above. A proposed mechanism for the reaction, which involves the free radicals HO⋅ and HOO⋅ , is represented by the three equations below. H2O2 → 2 HO⋅ (slow) H2O2 + HO⋅ → H2O + HOO⋅ (fast) HOO⋅ + HO⋅ → H2O + O2 (fast) a. Write the rate law consistent with the proposed mechanism above. b. The rate of the decomposition reaction was studied in an experiment, and the resulting data were plotted in the graph below. Using the graph, determine the time, in hours, needed for the concentration of H2O2 to change from i. 1.50 M to 0.75 M ii. 0.80 M to 0.40 M AP Chemistry Page 11 of 17 Test Booklet 5.11 Catalysis c. The experimental data are consistent with the proposed mechanism. Explain. An electrochemical cell based on the decomposition of H2O2 can be constructed based on the halfreactions in the table below. d. Calculate the value of the standard cell potential, E°, for the cell. e. Indicate whether ΔG° for the decomposition reaction is greater than 0, less than 0, or equal to 0. Justify your answer. f. The decomposition of H2O2(aq) is slow at 298 K, but a suitable catalyst greatly increases the rate of the decomposition reaction. i. Draw a circle around each of the quantities below that has a different value for the catalyzed reaction than for the uncatalyzed reaction. Keq ΔG° ΔH° Ea ii. For any quantity that you circled above, indicate whether its value is greater or less for the catalyzed reaction than for the uncatalyzed reaction. Explain why. Please respond on separate paper, following directions from your teacher. Page 12 of 17 AP Chemistry Test Booklet 5.11 Catalysis 8. The slowest step in a reaction mechanism requires the collision represented above to occur. Which of the following most likely indicates how the addition of a solid catalyst could increase the rate of the reaction? (A) The catalyst could change the reaction from second order to third order. 9. (B) The catalyst could increase the particles’ speed, thereby increasing the value of the rate constant, . (C) The catalyst could decrease the particles’ speed, making it less likely that the particles will rebound without reacting when they collide. (D) The catalyst could adsorb one of the particles, making a successful (reaction-producing) collision with the other particle more likely. O3 (g) + O(g) → 2 O2(g) The decomposition of O3(g) in the upper atmosphere is represented by the equation above. The potential energy in the upper atmosphere is diagram for the decomposition of O3(g) in the presence and absence of NO(g) is given below. Which of the following mechanisms for the catalyzed reaction is consistent with the equation and diagram above? AP Chemistry Page 13 of 17 Test Booklet 5.11 Catalysis (A) 2 O3(g) + 2 NO(g) → 4 O2(g) + N2(g) slow O3(g) + NO(g) → NO2(g) + O2(g) slow (B) NO2(g) + O(g) → NO(g) + O2(g) fast 10. (C) NO2(g) + O3(g) → NO(g) + 2 O2(g) slow NO(g) + O(g) → NO2(g) fast (D) NO2(g) + O(g) → NO3(g) slow NO3(g) + O3(g) → NO2(g) + 2 O2(g) fast To catalyze a biochemical reaction, an enzyme typically (A) drives the reaction to completion by consuming byproducts of the reaction (B) binds temporarily to reactant molecules to lower the activation energy of the reaction (C) dissociates into additional reactant molecules, thereby increasing the reaction rate (D) decomposes and releases energy to increase the number of successful collisions between reactant molecules 11. N2O(g) + CO(g) → N2(g) + CO2(g) The rate of the reaction represented above increases significantly in the presence of Pd(s). Which of the following best explains this observation? (A) Pd increases the activation energy of the reaction. (B) Pd absorbs the heat produced in the reaction. (C) One of the reactants binds on the surface of Pd, which introduces an alternative reaction pathway with a lower activation energy. (D) One of the products binds on the surface of Pd, which increases the reaction rate by decreasing the concentration of products in the mixture. Page 14 of 17 AP Chemistry Test Booklet 5.11 Catalysis 12. The diagram above illustrates how the reaction occurs on the surface of . This bond is The rate of this reaction is determined by the amount of energy required to break the bond in weakened when is adsorbed on . Based on this information, which of the following provides the best reason for the use of for the synthesis of ? (A) It promotes the proper orientation of and atoms to form new (B) It provides a reaction path with a lower activation energy. (C) It forms a bond between and bonds. that is stronger than the bond in (D) It decreases the frequency of collisions between and . . . The following questions relate to the below information. XY2 → X + Y2 The equation above represents the decomposition of a compound XY2. The diagram below shows two reaction profiles (path one and path two) for the decomposition of XY2. AP Chemistry Page 15 of 17 Test Booklet 5.11 Catalysis 13. Which of the following most likely accounts for the difference between reaction path one and reaction path two? (A) A higher temperature in path one (B) A higher temperature in path two (C) The presence of a catalyst in path one (D) The presence of a catalyst in path two 14. The hydrolysis of sucrose is represented by the chemical equation above. This reaction is extremely slow in aqueous solution. However, when sucrase is added as shown in the diagram, the rate of the reaction is about 6,000,000 times faster. Based on this information, which of the following best explains the large increase in the rate of hydrolysis that occurs with the addition of sucrase? 15. (A) The reaction proceeds through a different reaction path with a lower activation energy in which the sucrasesucrose complex is formed as an intermediate. (B) The addition of the sucrase reduces the number of effective collisions between the water and sucrose molecules. (C) The reaction proceeds through a different reaction path that is independent of temperature and concentration. (D) The addition of the sucrase decreases the number of sucrose molecules that have the minimum energy required to overcome the activation energy barrier. The role of a catalyst in a chemical reaction is to Page 16 of 17 AP Chemistry Test Booklet 5.11 Catalysis (A) decrease the amount of reactants that must be used (B) lower the activation energy for the reaction (C) supply the activation energy required for the reaction to proceed (D) increase the amounts of products formed at equilibrium (E) increase the entropy change for the reaction AP Chemistry Page 17 of 17