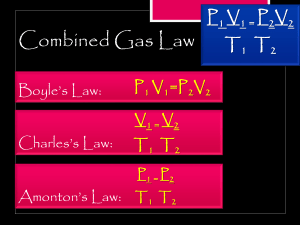
دبلوم تمريض مساق الكيمياء العامة chapter 7 Gases Jannat Azarah Properties of Gases That gases are fluid, compressible substances. All gases behave according to the following characteristics in the kinetic molecular theory (KMT): ■ Gas particles expand to assume the volume and shape of their container. تمتد جزيئات الغاز لتأخذ شكل وحجم الوعاء الذي توضع به ■ The volume of gas particles is assumed to be negligible. حجم جزيئات الغاز صغير جداً ويمكن إهماله ■ Gas particles are in constant motion. جزيئات الغاز لها حركة مستمرة ■ Gas particles mix evenly and completely when confined in the same container. متساو وبشكل كامل عندما تكون محصورة في نفس تختلط جزيئات الغاز بشكل ٍ الوعاء ■ Gas particles collide with each other; they do not attract or repel each other, and they do not exert a force on each other. وال، جزيئات الغاز تتصادم مع بعضها البعض ؛ وال يحدث بينها تجاذب أو تنافر يمارسون قوة على بعضهم البعض ■ The average kinetic energy of gas molecules is proportional to the temperature (in Kelvin) of the gas. .)متوسط الطاقة الحركية لجزيئات الغاز متناسب مع درجة حرارة الغاز (بالكلفن ■ The higher the gas temperature, the higher the kinetic energy. . زادت الطاقة الحركية، كلما ارتفعت درجة حرارة الغاز ■ The most common atmospheric pressure unit used by meteorologists is inches of mercury in Hg (760 mmHg = 29.92 in Hg). وحدة الضغط الجوي األكثر شيوعًا التي يستخدمها خبراء األرصاد الجوية هي • Because gases are compressible, they exert pressure on their surroundings. فإنها تمارس ضغ ًطا على المحيط، ألن الغازات قابلة لالنضغاط • Pressure is the force that is exerted over a unit area. For example: • the atmosphere exerts a pressure known as atmospheric pressure. الغازات الموجودة في الغالف الجوي تمارس ضغطا ً على سطح األرض يعرف بالضغط الجوي كلما ارتفعنا إلى أعلى قل الضغط • The Earth’s atmosphere is a function of the location and the weather conditions, and it decreases with a higher altitude. الغالف الجوي لألرض هو دالة في الموقع والظروف الجوية حيث أنه يقل مع المرتفعات • The unit of pressure commonly used in chemistry is the atmosphere (atm). • The standard atmosphere is 1 atm or a measurement of 760 millimeters of mercury (mm Hg or torr) on a manometer. • 1 atm = 760 mm Hg = 760 torr = 101,325 Pa (pascal or N/m2) • Example: Covert 830 mm Hg to atmospheres The gases Laws • Properties of gases that change are • • • • pressure (P), temperature (T), volume (V), and the number of moles (n). • Several laws relate these properties: 1-Boyle’s Law 2-Charles’s Law 3- Avogadro’s Law 4-Gay-Lussac’s Law 1-Boyle’s Law (at constant temperature): • The volume of a gas (maintained at constant temperature) decreases as its pressure increases (P α1/V): العالقة عكسية في حال ثبات درجة الحرارة.. كلما زاد الضغط قل الحجم P1V1 = P2V2 2- Charles’s Law (at constant pressure): • The volume of a gas (maintained at constant pressure) increases directly with an increase in its Kelvin temperature (V αT): 3- Gay-Lussac’s Law (at constant volume): The pressure of a gas (maintained at constant volume) increases with an increase in its Kelvin temperature (P αT): 4- Avogadro’s Law (at constant T and P): • The volume of gas increases with the number of moles of gas present at constant temperature and pressure.(V αn) The standard temperature and pressure (STP) The standard temperature and pressure (STP) condition is achieved at: • 273.15 K • 1 atm (760 torr) • 1 mole (or 6.02 x 1023 particles or molecules) • volume of 22.4 liters (molar volume at STP). Example: A 2.0 L sample of helium gas is at 25° C. What is the volume of the helium if the sample is heated to 75° C at constant pressure? Solution: Temperature and volume changing at constant pressure is Charles’s Law. First, all gas law problems require the use of Kelvin temperatures (K = °C + 273.15). The equation is then solved for V2 (because it is the second temperature that is unknown). • Combining Boyle’s Law, Charles’s Law, and GayLussac’s Law forms a combined gas law equation. • The combined gas law equation can be used when temperature, pressure, and volume are changing. • Essentially, this equation can replace the three individual law equations, and if one of the properties is constant, it can be crossed out and ignored. • Example: • On a warm spring day, an automobile tire with a volume of 82.5 L has a pressure of 2.18 atm (32 psi) at 24° C. After driving several hours, the temperature of the tires increases to 49° C and the pressure gauge shows 2.32 atm (34 psi). What is the new volume of the tire? Ideal Gas Law • Under normal conditions, most gases have a similar behavior. • The ideal gas law equation is used to calculate a variable at a specific point in time. The ideal gas law is a combination of the earlier laws: هو قانون يجمع المتغرات األربعة في معادلة واحدة شروط الغاز المثالي عدم وجود قوى تجاذب وتنافر بين جزيئات الغازات ًحجم الغاز صغير جدا The ideal gas law can also be modified to calculate • 1-mass (m) in grams. • 2-molar mass (Mm) in moles per gram. • 3- density (d) in grams per liter. Example: • It is a mistake to say that humid air is denser than dry air (it’s actually the liquid water condensing on you that makes the air “feel” denser). Because water mainly replaces nitrogen gas in the atmosphere, calculate the density of water vapor and nitrogen gas at STP So nitrogen is 50% denser than water. Note that water is a very small percentage of the atmosphere, and humid air is only a fraction denser than dry air Dalton’s Law of Partial Pressures • In a mixture of gases, individual gases behave independently so that the total pressure is the sum of partial pressures ينص قانون دالتون على أن الضغط الكلي لمخلوط غازات يساوي مجموع الضغوط الجزئية للغازات المكونة للمخلوط The partial pressures are also directly proportional to the molar amount or percentage of the component. تتناسب الضغوط الجزيئية طرديا ً مع الكتلة المولية أو مع النسبة المئوية لمكونات الغاز Example: Nitrogen is ~78% of the atmosphere and oxygen gas is ~21% of the atmosphere. What is the partial pressure for oxygen gas and nitrogen at STP (760 mm Hg)? Solution Pnitrogen gas = 760 mm Hg * 0.78 = 590 mm Hg Poxygen gas = 760 mm Hg * 0.21 = 160 mm Hg Example: In a balloon, the partial pressure of helium was 3.4 atm and nitrogen was 1.2 atm. What was the total pressure? Solution: Ptotal = sum of the partial pressures = 3.4 atm + 1.2 atm = 4.6 atm Effusion and Diffusion التدفق واالنتشار Effusion is the passage of a gas through a tiny hole usually into a chamber of lower pressure. هو مرور الغاز عبر ثقب صغير إلى منطقة ذات ضغط منخفض Thomas Graham experimentally determined that the rate of effusion is inversely proportional to the square root of the molecular mass. Graham’s law of diffusion states: .تجريبا ً قرر جراهام أن معدل التدفق يتناسب عكسيا ً مع الجذر التربيعي للكتلة المولية Effusion and Diffusion Diffusion is simply the mix of gases. Graham’s Law can be modified to approximate the distance traveled by a gas to mix: يمكن تعديل قانون جراهام لتقدير المسافة التي.هو ببساطة مزيج من الغازات :يقطعها الغاز لخلطه Example: Ammonia gas and hydrogen chloride gas readily come together to form ammonium chloride: NH3(g) + HCl(g) S NH4Cl(s). What is the diffusion ratio for these components? Solution: 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑒𝑑 𝑏𝑦 𝑔𝑎𝑠 𝑁𝐻𝑠 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑒𝑑 𝑏𝑦 𝑔𝑎𝑠 𝐻𝐶𝑙 = molar mass of HCl molar mass of NHs = 236.458 = 1.462 217.034 That means that the ammonia will travel nearly 1.5 times faster than the HCl gas. Real Gases Law A common mathematical equation used for real gases is the van der Waals equation. • عند الضغوط العالية جداً يكون الغاز غير مثالي • عند درجات الحرارة المنخفضة جداً يكون الغاز غير مثالي • تتقارب الجزيئات فتزيد القوى النسبية بين جزيئات الغاز وتزيد الكثافة فيتم تعديل قانون الغاز المثالي إلى قانون الغاز الحقيقي The van der Waals equation corrects the ideal • pressure and ideal volume with known constants a and b, respectively, for individual substances.