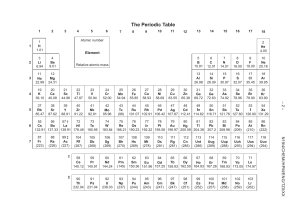
Empirical formula Name: ........................................................Group: ……….. Date: ………………. 1) What is the empirical formulae of a compound containing 34.5% copper, 30.5% iron and 34.9% sulfur by mass? …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… ……………………………………………………………………………………………………(3) 2) An organic compound P containing carbon, hydrogen and oxygen only was burnt in excess oxygen. 0.36g of the compound was combusted producing 0.88g of carbon dioxide and 0.36g of water. If the molar mass of compound P is 72, what is the empirical and molecular formula of P? …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… ……………………………………………………………………………………………………(7) Teacher Comments Markscheme /10