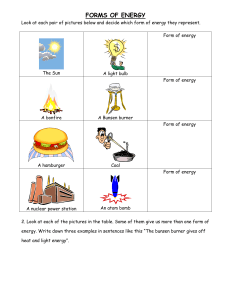
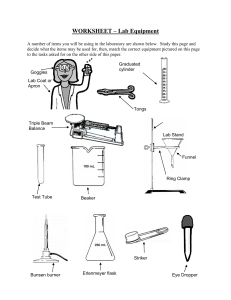
Orientation Day 2021 Welcome to Greenwood College! Write your name here: __________________________________ You are in a Science room. This room is referred to as Science room ____________. Today I am your Science teacher. My name is ___________________. In Science we teach you all about safety and risk management. Look around the room. See what hazards could possibly occur. Write down 3 safety rules that we need to follow when in a Science laboratory. 1. __________________________________________________ 2. __________________________________________________ 3. __________________________________________________ Observations Careful observation is a very important skill to have as a Science student. Today you will be observing and also carrying out a few different experiments. Your task is to record all of your observations. It is very important to follow all of the safety instructions. If you do not understand then please ask as there are lots of people who are able to help. Safety always comes first. Demonstration – Hydrogen Balloon What type of matter (solid, liquid, gas) was in the balloon? ___________ This demonstration was an example of a chemical reaction. What evidence do we have that a chemical reaction occurred? ______________________________________________________ In a chemical reaction a new substance is formed. We can represent a chemical equation with the following general equation. Reactants Products The 2 substances that reacted in this chemical reaction were the reactants. The reactants in this chemical reaction were ______________ and _____________________. The new substance that was formed in this chemical reaction was the product. The product in this chemical reaction was ___________________. We can now write a word equation for this reaction. ________________ + ______________ __________ Who thinks they can write a chemical equation using symbols? Have a go! ____________ + ____________ _____________ Over to you If you look around the room you will see that we have a few different workstations. Each workstation will have an activity to complete. Complete your activity booklet at each workstation. Please stay with your group. I will instruct you when to move to your next station. Workstation 1 – Microscopes – Viewing Euglena You will be viewing Euglena using the microscope. Euglena are commonly found in pond water. They are tiny, and are made up of only one cell. They are referred to as single celled organisms. I wonder what Kingdom they belong to? You will learn about this in Biology next year. Task: View the Euglena under the microscope. Write down your observations for each of the different magnifications. The Year 10 helper will assist you with this. Magnification Observation of euglena X 40 X 100 X 400 Workstation 2 A Bunsen burner is a very common piece of equipment used in Science. Today you are going to learn how to safely light a Bunsen Burner. Next year you will get the chance to earn your Bunsen burner licence when you do Chemistry. Steps to safely light a Bunsen burner 1. Make sure your workspace is not cluttered and long hair is tied back 2. Connect the Bunsen burner to the gas tap 3. Use the collar to close the air hole 4. Light the match. Hold it over the top of the Bunsen burner 5. Turn on the gas at the tap 6. Light the Bunsen burner. What colour is the flame? ____________ 7. Use the collar to open the air hole. What colour is the flame? __________________ Workstation 3 A chemical reaction between a metal and an acid – “Pop Test” 1. Add a piece of magnesium ribbon to a test tube. 2. Using a dropper bottle add hydrochloric acid so that the magnesium ribbon is covered. 3. Use a stopper to seal the end of the test tube. 4. Carefully, light a match. Take the stopper off and hold the match over the end. You will need to do it quickly! 5. Ask the Year 10 helper to show you how. List 3 observations 1. __________________________________________________ 2. __________________________________________________ 3. __________________________________________________ Workstation 4 – Acids and Bases 1) Place one squirt of the acid into the test tube. Write down your prediction of what colour you think it will be. Add the universal indicator. Observe what happened. 2) Do the same for the base. Prediction Observation Acid Base Once finished. 1. Assist your group leader in tidying up the workstation you finished at. 2. Put your safety glasses away. 3. Wash your hands. What can you remember? Test yourself on the quiz below. 1) Name an important rule when working in a science room ____________________________________________________ ____________________________________________________ 2) Name a single celled organism ____________________________________________________ 3) State 2 pieces of evidence that a chemical reaction has occurred i. ___________________________ ii. ___________________________ 4) Hydrogen gas is flammable. What does this mean? ______________________________________________________ We hope that you have had a great day and enjoyed this lesson. All the Science teachers at Greenwood College looks forward to seeing you in Science next year!