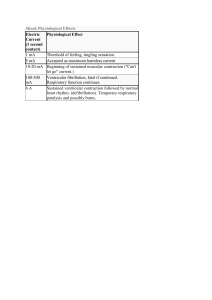
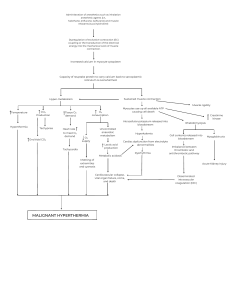
OMoARcPSD| 13702040 lOMoAR cPSD| 13702040 Pacemaker potential: Slow depolarization: brings membrane potential to a threshold, where the action potential occurs • • K permeability steadily decreases due to previous AP (repolarization) Inward, depolarizing Na current (F-type channels) • Ca channel opens briefly to add final depolarizing boost to pacemaker potential (T-type Ca channels) Excitation-Contraction Coupling: **process of this is in the cardiac muscle section of muscle phys. • Amount of cytosolic Ca 2+ increase during excitation is a determinant of cardiac muscle contraction strength • In cardiac muscle, amount of Ca released from a single action potential (from sarcoplasmic reticulum) in cardiac muscle during rest isn’t usually sufficient to saturate all troponin sites in order to initiate cross-bridge cycling • More Ca leads to more/ stronger muscle contraction Heart Refractory Period: • Cardiac muscle is incapable of summation Absolute refractory period: period during and following an action potential when an excitable membrane can’t be re-excited. • Allows for controlled contraction of heart muscles Skeletal muscles (and neurons) deactivate by the closing of Na+ channels which happens very quickly and allows for very quick regeneration of a second action potential (frequency of AP is proportional to stimulus strength). • Because of prolonged/ depolarized plateau in cardiac muscle AP, absolute refractory period lasts almost as long as the whole contraction which controls rate of contraction lOMoAR cPSD| 13702040 Mechanical Events of the Cardiac Cycle: Two major phases: systole: ventricular contraction and blood ejection lOMoAR cPSD| 13702040 Isovolumetric ventricular contraction: (first part of systole) ventricles are contracted but all valves in the heart are closed so no blood ejection • • Constant ventricular volume Tension caused by pressure on ventricular walls • Similar to isometric skeletal muscle contraction: muscles don’t shorten Ventricular ejection: aortic and pulmonary valves open due to pressure in ventricles exceeding that in the aorta and pulmonary trunk Stroke volume (SV): blood volume ejected from each ventricle during systole Systole turns into diastole when the ventricular contraction stops and pulmonary and aortic valves close diastole: ventricular relaxation and blood filling • Each cycle lasts about 0.8 sec. (0.3 systole and 0.5 diastole) Isovolumetric ventricular relaxation: the AV valves are closed so volume remains constant Ventricular filling: AV valves open and blood flows into the ventricles from the atria • Atrial contraction occurs at the end of diastole • About 80% of ventricular filling occurs before atrial contraction Left Ventricle and Aorta: • Typical systolic and diastolic pressures are 120 and 80 mmHg lOMoAR cPSD| 13702040 Mid-to-Late Diastole (ventricle filling: relaxed state) 1. Left atrium and ventricle are relaxed, atrial pressure is higher than ventricular pressure (because it is filling with blood from the veins) 2. AV valve is held open by the pressure difference and blood entering atrium from pulmonary veins continues into the ventricle lOMoAR cPSD| 13702040 3. Aortic valve remains closed because aortic pressure is higher than ventricular pressure 4. Aortic pressure decreases throughout diastole because blood moves out of the arteries into the veins 5. Ventricular pressure is increasing because blood enters ventricle from the atrium 6. Towards the end of diastole, SA node discharges and atria depolarize 7. Contraction of atrium causes increase in atrial pressure 8. Increased atrial pressure forces through the "atrial kick” (small addition of blood) 9. End-diastolic volume: end of ventricular diastole Systole: (ventricular contraction: blood ejection) 10. From the AV node, depolarization passes into and through the ventricle and triggers ventricular contraction 11. As contraction occurs, ventricular pressure rapidly increases (to exceed atrial pressure 12. This pressure increase causes the AV valve to close (prevents back flow or “regurgitation") 13. Aortic pressure is still not overcome by the ventricular pressure and all valves are closed. Backwards bulging of the closed AV valves causes an upward deflection in atrial pressure wave (isovolumetric ventricular contraction) 14. Ventricular pressure exceeds aortic pressure 15. Pressure causes aortic valve to open and ejection begins 16. Ejection of blood is rapid at first and then gradually slows down 17. End-systolic volume: blood remaining in the ventricle after ejection **Stroke Volume (SV) = End-Diastolic Volume (EDV) - End-Systolic Volume (ESV) 18. Aortic pressure goes up along with ventricular pressure because aortic valve offers little resistance to flow so it all acts as one "tube" 19. Peak ventricular and aortic pressures are reached before the end of ejection 20. Force reduction is because of reduced rate of blood ejection during the end of systole 21. Volume and pressure of aorta decrease due to less blood Early Diastole (ventricular relaxation: blood filling) 22. T wave of ECG corresponds to ventricular repolarization 23. As ventricles relax, ventricular pressure decreases below aortic pressure which forces aortic valve to close Dicrotic notch: rebound of aortic pressure caused by elastic recoil of aorta and blood against the valve lOMoAR cPSD| 13702040 24. AV valve remains closed because ventricular pressure is still higher than atrial pressure. Then there is a brief period where all valves are closed (isovolumetric ventricular relaxation) 25. Ventricular pressure decreases below aortic pressure 26. Pressure change results in opening of the AV valve 27. Venous blood from atrium flows into the ventricle 28. Blood flow rate is enhanced during initial filling phase by rapid decrease in ventricular pressure due to recoil of ventricle (expanding past normal size) after systole from previous contraction: causes rapid decrease in pressure • Energy is stored in the myocardium during contraction, and its release during relaxation helps with filling • Most filling of the ventricle occurs in the early diastole phase to ensure that the contraction/ relaxation cycle is not disrupted by an elevated heart rate (i.e. during exercise) Pulmonary Circulation Pressures: lOMoAR cPSD| 13702040 • • Typical systolic and diastolic pressures are 25 and 10 mmHg Referred to as “low-pressure” system • Right ventricular wall is much thinner than the left • Stroke volumes of the two ventricles are the same Heart Sounds: When listening through a stethoscope: • First “thud” is closing of the AV valves (systole) • Second, louder “thud”is the closing of the aortic and pulmonary valves (diastole) Laminar flow: smooth concentric layers of blood Turbulent flow: caused by blood flowing rapidly in usual direction through abnormally narrowed valve to cause disruption of laminar flow (causing cells to change directions) Stenosis: narrowed valves Insufficiency: blood flow backwards through damaged valve Septal defect: blood flowing between two atria or two ventricles The Cardiac Output: the volume of blood each ventricle pumps as a function of time (L/min) lOMoAR cPSD| 13702040 CO = HR (bpm) x SV Control of HR: Chronotopic effects: parasympathetic neurons cause HR to decrease, whereas sympathetic neurons cause the HR to increase Nerves that alter HR: • Sympathetic stimulation: increase in Na permeability : faster depolarization • Parasympathetic input: increased permeability to K : hyper-polarization Hormones affecting HR: lOMoAR cPSD| 13702040 • Epinephrine: main hormone secreted by the adrenal medulla speeds up HR by acting on beta-adrenergic receptors in SA node (same as those affected by norepinephrine released by neurons) Dromotropic effects: • sympathetic stimulation increases conduction velocity through entire cardiac conducting system • Parasympathetic stimulation decreases the rate of spread of excitation through the atria and AV node Control of SV: Changes in force during ejection of the stroke volume: 1. Changes in end-diastolic volume (volume of blood in the ventricles just before contraction or preload) lOMoAR cPSD| 13702040 **SV increases as end diastolic volume increases (Frank- Starling mechanism): at any given heart rate, an increase in venous return- the flow of blood from the veins into the heart automatically forces an increase in cardiac output by increasing end-diastolic volume and therefore SV • Length-tension relationship causes the F-S mechanism because end-diastolic volume is a major determinant of how stretched the ventricular sarcomeres are just before contraction • Difference between length-tension relationship in cardiac vs. skeletal muscles is normal point for cardiac muscle is in a resting individual is not at its optimal length for contraction • Greater filling causes additional stretching of cardiac muscle fibers and increases force of contraction 2. Changes in magnitude of sympathetic nervous system input to the ventricles lOMoAR cPSD| 13702040 lOMoAR cPSD| 13702040 • Sympathetic neurotransmitter NE acts on Beta-adrenergic receptors to increase ventricular contractility- strength of contraction at any given end-diastolic volume Inotropic effects: caused by extrinsic factors that increase the force of contraction at a given enddiastolic volume Contractility is quantified through ejection fraction (EF) = SV / EDV • Averages between 50%-75% under resting conditions in a healthy heart • Causes contraction and relaxation of ventricles more quickly Adrenergic receptors activate a G-protein coupled cascade: causes production of cAMP and protein kinase Proteins involved with excitation-contraction coupling are phosphorylated by the kinase which enhances contractility. Proteins include: 1. L-type Ca channels 2. RyR receptors and proteins associated proteins in the sarcoplasmic reticulum membrane 3. Thin filament proteins (troponin) 4. Thick filament proteins associated with cross-bridges 5. Proteins involved in pumping Ca to the sarcoplasmic reticulum lOMoAR cPSD| 13702040 Cytosolic Ca concentration increases more quickly during excitation and returns back to its resting value quickly too **parasympathetic system has negligible effects on ventricular contractility 3. Changes in after load (arterial pressure against which the ventricles pump) • Increased arterial pressure reduces SV • Greater the load, the less contracting muscle fibers can shorten at a given contractility lOMoAR cPSD| 13702040 The Vascular System: • Every tube has a layer of endothelial cells (endothelium) lining the flowing blood (smooth) lOMoAR cPSD| 13702040 • By the time the blood reaches the venous system, blood flow rate decreases due to resistance from the body lOMoAR cPSD| 13702040 Arteries: • • Arteries have thick walls (many more layers of tissue (smooth muscle) around the endothelium) Arteries can be thought of as elastic tubes Main functions of the Aorta: • Serve as a low-resistance tube conducting blood to various organs • Acts as a pressure reservoir for maintaining blood flow through tissues during diastole Arterial Blood Pressure: Compliance: how easily a structure stretches: Compliance = change in volume / change in pressure • • • Only about a third of of the SV leaves the arteries during systole, which increases arterial pressure As blood leaves arteries, arterial volume and pressure slowly decrease Next ventricular contraction occurs while artery walls are still stretched by the remaining blood so arterial pressure never hits 0 lOMoAR cPSD| 13702040 Systolic pressure (SP): max arterial pressure reached during peak ventricular ejection Diastolic pressure (DP): minimum arterial pressure occurs just before ventricular ejection begins Pulse pressure: difference between systolic and diastolic pressure **felt in the wrist or neck: expansion of the blood vessel is what we feel Factors determining pulse pressure: • • Stroke volume Speed of ejection of SV • Arterial compliance Mean Arterial Pressure (MAP): average pressure during the cycle lOMoAR cPSD| 13702040 Arterioles: Major functions: • Arterioles in individual organs are responsible for determining the relative blood flows to an organ at any given MAP • All together, arterioles are the major factor in determining MAP As blood flows from the arteries into the arterioles, what determines which arteriole the blood will flow into? • • Differences in flow are determined by differences in the resistance to flow offered by each tube Differences in resistance are solely due to differences in radii of each tube • Widest tubes have lowest resistance • Arterioles are the major sites of resistance due to their small radii • MAP decreases from around 90 to 35 mmHg as blood flows through the arterioles lOMoAR cPSD| 13702040 Vasodilation: smooth muscle in arterioles relax and cause radius to increase Vasoconstriction: smooth muscle in arterioles contracts and causes radius to decrease How is resistance altered? • Intrinsic tone: contraction independent of any neural, hormonal, or paracrine input (spontaneous contraction) • Also called basal tone: sets baseline level of contraction that can be increased or decreased by external signals (NT and hormones) • Signals act by inducing changes in cytosolic Ca concentration or vascular smooth muscle cells • Mechanism controlling vasodilation/ vasoconstriction fall into two categories: lOMoAR cPSD| 13702040 Local control: mechanisms independent of nerves or hormones • Can be effected by changes in para/ autocrine agents Active Hyperemia: increased blood flow when metabolic activity increases • • Direct result of arteriolar dilation in more active organ/tissues Most highly developed in skeletal, cardiac muscle, and glands • Arteriolar smooth muscle relaxes in active hyperemia due to chemical changes in extracellular fluid surrounding arterioles These chemical changes vary with effectors but some possible changes are: • Local oxygen concentration decreases (used during ATP production) Increases in: • • CO2 (end product of oxidative metabolism) H+ ions (from lactic acid) • • Adenosine (breakdown of ATP) K+ ions (from AP repolarization) • • Eicosanoids (breakdown product from phospholipids Osmotically active breakdown • Bradykinin: peptide generated from kininogen, with help of enzyme kallikrein, from precursory prekallikrein secreted from liver • Nitric oxide: gas released by endothelial cells **cause vasodilation in active hyperemia Flow autoregulation: change in resistance is in the direction of maintaining blood flow nearly constant despite pressure change lOMoAR cPSD| 13702040 • When decrease in arterial pressure reduces blood flow to an organ, oxygen supply goes down • Extracellular fluid concentrations of above molecules • Vasodialations of active hyperemia and flow auto regulation in response to low arterial pressure involve similar metabolic mechanisms Flow auto regulation can also cause arterial pressure increases: • Due to increase in pressure • Increase in oxygen concentration: causes vasoconctriction to maintain steady flow despite increased pressure • Increased arterial pressures causes increased wall stretch, also causes arteriolar smooth muscle to contract (myogenic response): caused by Ca movement Reactive hyperemia: when an organ or tissue has had its blood supply completely occluded, a profound transient increase in its blood flow occurs if flow is reestablished • Extreme form of autoregulation • When there is no blood flow, arterioles dilate (pressure decrease so blood can flow in) Local response to injury: • Cause eicosanoids and other substances to be released locally from cells • These substances make arteriolar smooth muscle relax and dilate in injured area to increase blood flow to that area (inflammation) Extrinsic Controls: Sympathetic neurons: • Sympathetic post-ganglionic neurons innervate most arterioles • Mainly release NE which binds to alpha-adrenergic receptors on vascular smooth muscle to cause vasoconstriction Control of sympathetic neurons to arterioles can be used to vasodilate • Discharge of sympathetic neurons cause some degree of tonic constriction (vary from organ to organ) • Dilation is achieved by decreasing rate of sympathetic activity to below the basal level lOMoAR cPSD| 13702040 Function: • Reflexes that serve whole-body needs • Common reflex is regulating arterial blood pressure by influencing arteriolar resistance throughout the body • Redistributing bloodflow to achieve specific function Parasympathetic neurons: • Most blood vessels receive sympathetic and not parasympathetic innervation Noncholinergic, nonadrenergic, Autonomic neurons: • Release vasodilator (nitric oxide) Hormones: • Epi /NE bind to alpha-adrenergic receptors on arteriolar smooth muscle and cause vasoconstriction • Binding of Epi to Beta2 (located in the same region as Alpha but fewer) receptors causes muscles to relax Angiotensin II: hormone that constricts arterioles Vasopressin: causes vasoconstriction as well Atrial natriuretic peptide: vasodilator Endothelial Cells and Vascular Smooth Muscle: lOMoAR cPSD| 13702040 Endothelial cells (in response to substances acting indirectly) secrete paracrine agents that diffuse to adjacent vascular smooth muscle and either relax or contract, which results in vasodilation/ vasoconstriction Important paracrine vasodilator released by endothelial cells is nitric oxide • Nitric Oxide is continuously released in significant amounts in arterioles and contributes to vasodilation in basal state • Its secretion also increases in response to large number of chemical mediators involved in reflex/ local control systems Another vasodilator is prostacyclin: little basal secretion of PGI2 but can increase in response to inputs Endothelin-1: important vasoconstrictor paracrine agent released by endothelial cells in response to certain stimulus • Can reach a high enough concentration in blood to also function as hormone in addition to paracrine agent, causing arteriolar vasoconstriction Arteriolar Control in Specific Organs: lOMoAR cPSD| 13702040 lOMoAR cPSD| 13702040 Capillaries: • At any given moment, about 5% of blood is in capillaries: performing exchange of nutrients, metabolic end products, and cell secretions Angiogenesis: capillary growth Endothelial cells are involved in building of new capillary networks by cell locomotion and cell division Angiogenic factors: stimulate angiogenesis: secreted locally by various tissues and cells Anatomy: lOMoAR cPSD| 13702040 Capillary: (varies from organ to organ) thin-walled tube of endothelial cells one layer thick, resting on a basement membrane, without any surrounding smooth muscle or tissue • They can also have a second set of cells that surround the basement membrane that restrict some substances from passing through Intercellular clefts: narrow water-filled spaces lOMoAR cPSD| 13702040 • Endothelial cells generally contain vesicles for end/exocytosis • Also form continuous fused- vesicle channels Metarterioles: vessels that connect arterioles to venules • • Blood enters capillaries through met arterioles sometimes Contain scattered smooth muscle cells • Capillary exits from a metarteriole is surrounded by a ring of smooth muscle called the precapillary sphincter, which relaxes or contracts in response to metabolic factors • More active, the more precapillary sphincters open Lipid-soluble substances can diffuse Ions and polar molecules diffuse through gaps/ channels Velocity of Capillary flow: lOMoAR cPSD| 13702040 • • Above: model that shows how the branching of tubular structure influences velocity Velocity of flows decreases as the sum of cross-sectional area of the tubes increase • Flow gets very slow in the capillary to allow time for material exchange Movement via diffusion is given by a gradient • Gradient caused by cell usage or production Flow across Capillary walls: starling forces: • • Bulk flow of protein-free fluid from plasma to interstitial fluid determines distribution of ECF All about fluid balance and no other factors lOMoAR cPSD| 13702040 lOMoAR cPSD| 13702040 Veins: Structure: • Large lumen • 1 way flow Function: • Low resistance conduits to return blood to heart • Volume reservoirs lOMoAR cPSD| 13702040 Venous Return: • Valves only allow blood to return towards heart • Anything that increases venous pressure or decreases atrial pressure will increase venous return Venous return is increased by: • • Venoconstriction (increase in SNA) Skeletal muscle pump • Respiratory pump: inspiration Lymphatic Vessels: lOMoAR cPSD| 13702040 Lymphatic system is composed of a network of lymph nodes and lymph vessels: • • Start at capillaries in peripheral tissues Contains highly- permeable vessels • Lymph: fluid derived from interstitial fluid • Lymphatic ducts empty into veins near right atrium Functions: • • Return fluid and plasma proteins to vasculature Immune funciton • Absorb fat from GI tract Integration of CV function: regulation of systemic arterial pressure: • Major cardiovascular variable being regulated is the mean arterial pressure in systemic circulation (MAP = CO x TPR) • **all changes in mean arterial pressure must be the result of changes in cardiac output and/or total peripheral resistance (not just some) • • CO = volume of blood pumped into arteries per unit time Major site of resistance in systemic arterial system are the arterioles • So total peripheral resistance is determined by total arteriolar resistance Summary of factors that determine systemic arterial pressure: lOMoAR cPSD| 13702040 Sequence of events by which a decrease in blood volume leads to decrease in mean arterial pressure: Hemorrhage: significant blood loss : decrease in MAP lOMoAR cPSD| 13702040 Baroreceptor reflexes: short term (seconds to hours) homeostatic adjustments to MAP • Effectors are mainly changes in activity of autonomic neurons supplying the heart and blood vessels, in addition to changes in secretion of hormones that effect MAP (Epi, angiotensin II, and vasopressin) Arterial Baroreceptors: "pressure sensors" • Two baroreceptors are located where the left and right common carotid arteries divide into two smaller arteries that supply head with blood • Artery wall is thin and is sensitive to stretching of contracting which is a direct indicator of blood pressure • The two carotid sinuses and aortic arch baroreceptor constitute the arterial baroreceptors (innervated by afferent neurons that provide input to the cardiovascular control center Medullary CV Center: primary integrating center for baroreceptor reflexes (highly interconnected neurons) • • Located in the medulla oblongata Neurons receive input from baroreceptors • Input determines AP frequency lOMoAR cPSD| 13702040 • Arterial baroreceptors increase their rate of discharge, the result is a decrease in sympathetic neuron activity, and increase in parasympathetic neuron activity • Decreased arterial pressure causes increase in Angiotensin II and Vasopressin secretion Operation of the Baroreceptor Reflex Arc: lOMoAR cPSD| 13702040 • Baroreceptors are short term, however if BP stays elevated for multiple days, baroreceptors adapt to this different BP, and decrease their firing frequency at any given pressure Hemorrhage and other Causes of Hypotension: lOMoAR cPSD| 13702040 Hypotension: low blood pressure • Immediate response to hypotension is baroreceptor reflex described above • Skin can become pale in time of hemorrhages because the blood is not needed in the skin cells as much as it is needed elsewhere (brain/ heart) *Another type of compensatory mechanism is interstitial fluid moves into the capillaries • Because decrease in BP and increase in arteriolar constriction will decrease capillary hydrostatic pressure (favoring absorption of interstitial fluid) Autotransfusion: blood loss and decreased blood volume are compensated for by the movement of interstitial fluid into the vascular system • Autotransfusion can restore blood volume to normal levels within 12-24 hours of moderate hemorrhage lOMoAR cPSD| 13702040 Shock: any situation in which a decrease in blood flow to organs and tissues damages them Hypovolemic shock: shock caused by decrease in blood volume secondary to hemorrhage or loss of fluid other than blood Low-resistance shock: due to a decrease in total peripheral resistance secondary to excessive release of vasodilators (allergy/ infection) Cardiogenic shock: due to extreme decrease in cardiac output from any of a variety of factors Hypertension: chronically increased systemic arterial pressure (above 140/90 mmHg) Left ventricular hypertrophy: adaptive increase in cardiac muscle, caused by the heart chronically pumping against an increased arterial pressure (after load) Stroke: blockage or rupture of a cerebral blood vessel (localized brain damage) lOMoAR cPSD| 13702040 Primary hypertension: uncertain cause Secondary hypertension: identified cause of hypertension Diuretics: increase Na and water loss in urine Heart Failure: occurs when the heart doesn’t pump an adequate cardiac output People with heart failure are separated into two categories: 1. Diastolic disfunction • Wall of the ventricle has reduced compliance • Reduced ability to fill adequately at normal diastolic filling pressures • Causes a reduced end-diastolic volume : reduced SV 2. Systolic dysfunction lOMoAR cPSD| 13702040 • Results from myocardial damage • Decrease in cardiac contractility : lower SV at any given end-diastolic volume Reduced cardiac output triggers an arterial baroreceptor reflex: afferent baroreceptors become less sensitive resulting in: (short term) • Heart rate increases (increases sympathetic innervation and decreased parasympathetic innervation) • Total peripheral resistance increases by increasing sympathetic activation of systemic arterioles and increased plasma concentration of angiotensin II and vasopressin (hormonal vasoconstriction) • Fluid retention is initially an adaptive response to decreased CO. As this progresses over longer time, as ventricle is distended with blood, force of contractility decreases, and fluid retention increases (can cause edema: accumulation of interstitial fluid) • Because capillaries drain via venules into the veins, so increased capillary pressure also increases filtration so there is more extra interstitial fluid rather than extra plasma Pulmonary edema: accumulation of fluid in the interstitial spaces of the lung or air spaces. • • Impairs pulmonary gas exchange Left ventricle fails to pump blood so blood volume in pulmonary circulation increases. • Causes capillary pressure to exceed normal pressures causing more filtration lOMoAR cPSD| 13702040 Hypertrophic Cardiomyopathy: condition that frequently leads to heart failure • Occurs in about 1/500 people • Characterized by an increase in thickness of the heart muscle (specifically the inter ventricular septum at the wall of the ventricle) • Disruption of orderly array of myocytes and conducting cells within the walls Hemostasis: The prevention of blood loss Hemostasis: stopping of bleeding **mechanisms are most effective in small vessels (arterioles, capillaries, and venules) • Venous bleeding leads to less blood loss because of low blood pressure in veins • If venous bleeding is internal, accumulation of blood in tissues may increase interstitial pressure enough to eliminate pressure gradient required for continues blood loss Hematoma: accumulation of blood in tissues occurs as result of bleeding from vessel • When a blood vessel is damaged, its immediate response is to constrict which slows blood flow in affected area (endothelial cells can often times stick together like glue) lOMoAR cPSD| 13702040 Stopping of further bleeding depends on two processes: 1. Formation of a platelet plug • • Minor damage Platelets adhere to collagen via an intermediary called von Willebrand factor (vWF): a • plasma protein secreted by endothelial cells and platelets vWF binds to exposed collagen molecules allosterically, and is then able to bind platelets • “Forms a bridge" between damaged vessel wall and platelets • Binding of platelets to collagen triggers platelets to release ADP and serotonin to assist locally and speed up metabolism in the area • Platelet activation: change in shape and surface proteins of platelets • • Platelet aggregation: new platelets adhere to old ones (+ feedback) which rapidly creates a platelet plug in the vessel Adhesion of platelets rapidly induces them to synthesize thromboxane A2: (acidic) • When released thromboxane A2 acts locally to promote further platelet aggregation lOMoAR cPSD| 13702040 Platelets contain a lot of actin and myosin which stimulated to interact in aggregated platelets • Causes compression and strengthening of platelet plug Fibrinogen: plasma protein that forms bridges between aggregating platelets Smooth muscle of damaged area contracts to build up pressure so there is less blood flow to the area • Vasoconstriction is the result of platelet activity and is mediated by thromboxane A2 Stopping platelet aggregation: • • Adjacent undamaged cells release prostacyclin (prostaglandin I2 (PGI2)) Acts as inhibitor of platelet aggregation • Adjacent cells also release nitric oxide which acts as vasodilator (also has inhibitor of platelet aggregation) 2. Blood coagulation (clotting) • Dominant hemostatic response • • Process of turning blood into a clot (solid gel : thrombus) Clot consists of mainly fibrin polymer • Clot occurs locally around platelet plug lOMoAR cPSD| 13702040 • Begins with exposure of blood to sub-endothelial tissue • Prothrombin must be converted to thrombin: Roles of Thrombin: 1. Cleaves fibrinogen into fibrin 2. Activates factor XIII to stabilize fibrin mesh 3. + feedback effect on clotting cascade Coagulation can be initiated by two pathways: 1. Intrinsic pathway: exposure of plasma proteins to sub-endothelial tissue 2. Extrinsic Pathway: Binding of Plasma protein to a protein located on the cell membrane of sub-endothelial cells lOMoAR cPSD| 13702040 • • Activation of extrinsic pathway initiates activation of intrinsic pathway Intrinsic pathway activation is very weak • Extrinsic pathway produces thrombin, but not enough to clot • However, thrombin from extrinsic pathway acts as + feedback to produce more thrombin through intrinsic pathway to supplement to be able to sufficiently clot Liver produces plasma proteins necessary for clotting cascade (indirect effect) • Requires vitamin K • Bile salts help GI tract absorb vitamin K lOMoAR cPSD| 13702040 Regulating Blood Coagulation: Blood Coagulation is regulated by many factors, all of which rely on endothelial cells • • Inhibition of platelet activation: (**shown above) release of prostacyclin and NO from endothelial cells which inhibit aggregation Inhibition of clot formation: same as inhibition of platelet activation • Factors that dissolve clots: plasmin (active form of plasminogen) dissolves fibrin lOMoAR cPSD| 13702040 Terms: Aspirin inhibits platelet cycloocygenase activity: inhibiting prostaglandin and thromboxane production (inhibit platelet aggregation) Oral anticoagulants and heparin interfere with clotting factors to prevent clotting Recombinant tissue plasminogen activator (t-PA) is a thrombolytic - dissolves blood clots after they are formed