Chemistry I Unit 4 Review: Atomic Structure & Nuclear Chemistry
advertisement

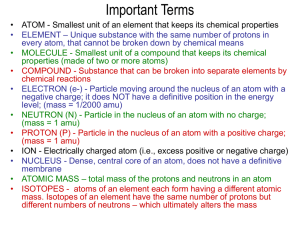
Chemistry I Unit 4 review 1. Describe how each of the following were discovered a. Electrons b. nucleus/protons 2. Describe the evidence used to disprove the plum pudding model of the atom 3. Describe the contributions of the following scientists a. Thomson b. Millikan c. Rutherford d. Dalton e. Bohr 4. Answer the following about isotopes: a. Carbon has several stable isotopes Carbon-12, Carbon-13, Carbon-14, and Carbon-15, The average atomic mass found on the periodic table is 12.01 amu. Which of Carbon’s isotopes is the most abundant in nature and how do you know? b. A sample contains atoms with 17 protons, and a range of neutrons from 17-19 neutrons each. i. What element is this sample? ii. What are 2 possible isotopes of this element? iii. Write the isotope notation for these isotopes 5. Mass laws a. Which of the mass laws is being described in each instance below? i. A sample of sodium chloride from the mineral Halite contains 1 atom of sodium and 1 of chlorine. Another sample from ocean water contains 1 atom of sodium and 1 atom of chlorine. ii. A 10 g sample of Magnesium oxide is decomposed by heat a mass of 5.9 g of solid magnesium remains the researcher determines that 4.1g of oxygen must have been released in the decomposition. iii. Sulfur and oxygen exist as 2 different compounds 1 contains a ratio of 1 atom of Sulfur to 2 atoms of oxygen while the other has a ratio of 1:3. 6. Using the law of definite proportions to solve the following: a. What mass of oxygen is present in a 55 g sample of NaHCO3 ? b. Determine the percent composition of C6H6O6 7. What is the percent of oxygen by mass in a sample of Copper oxide given that a 6.8 g sample of the copper oxide is heated and 5.4 g of copper remains 8. Using the band of stability: determine if the following isotopes are likely to be stable or unstable. a. 10p & 11 n b. 67 p & 40 n c. 100 p & 80 n 9. Write the nuclear decay equations for the following a. Alpha decay of Uranium-235 b. Beta decay of Nickel-65 c. Positron emission of Radium-255 d. Electron capture of Fluorine-20 10. Half life problems a. The half-life of Zn-71 is 2.4 minutes. If one had 100.0 g at the beginning, how many grams would be left after 7.2 minutes has elapsed? b. After 24.0 days, 2.00 milligrams of an original 128.0 milligram sample remain. What is the halflife of the sample? 11. Fission vs. Fusion a. Given each of the following determine if the statement refers to Fission or Fusion i. Used in nuclear power plants ii. Occurs in the sun iii. A larger nucleus divides to make a smaller nucleus iv. 2 Hydrogens atoms fuse to make a helium atom v. A critical mass is necessary to explode b. During fission, what type of particle is captured by a nucleus? 12. What are the 4 Fundamental Forces? a. Which is the strongest? Weakest? b. Which are important for stable nuclei? c. What holds protons and neutrons together in a nucleus? d. Is the strong nuclear force attractive, repulsive, or both? 13. Calculate the Average atomic mass of Chlorine given it has 2 naturally occurring isotopes Chlorine-35 with a mass of 34.97 amu with an abundance of 75.76% and Chlorine-37 with a mass of 36.97 amu with an abundance of 24.24%