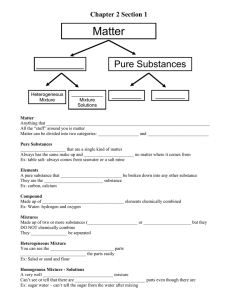
Name:_______________________________________________________ Date:_____________________________ Period: ______ Physical and Chemical Properties Lab Background Information: Matter, the “stuff” that makes up everything we see, can be classified in several manners. It can be found as a pure substance, an element or compound, or a mixture. Each of these will, in turn, have both physical and chemical properties. To investigate these properties of matter, a few definitions must be made. Pure substances can either be elements or compounds. Elements are anything found on the periodic table. As long as the matter is comprised only of that element, it is a pure substance. A chunk of copper, oxygen gas, a lead weight, and a diamond (pure carbon) are prime examples of a pure substance that is in an elemental form. A compound is also a pure substance made up of more than one element, as long as there are no impurities. Pure water, sodium chloride, calcium carbonate, and aluminum oxide, are examples of a pure substance in the form of a compound. Mixtures occur when two or more elements or compounds are mixed together. There are two types of mixtures, homogeneous and heterogeneous. Homogeneous mixtures are when the properties of the solution are the same throughout. Examples of this would be salt water, air, vanilla ice cream, and metal alloys like stainless steel. Heterogeneous mixtures are when the properties can change, depending on where you are looking in the solution. Examples of this would be and oil/water mixture, rocky road ice cream, and raw metal ores (metal mixed in with dirt and such). Each of these classifications of matter has both chemical and physical properties. A physical property is one that can be observed without altering the substance. That is to say, it remains unchanged after the observation. Examples of this include, but are not limited to, color, odor, phase changes (boiling, melting, and freezing points), solubility, miscibility, hardness, and density. Each of these is observable without creating a new substance. A chemical property is essentially whether or not the substance will react with another substance. For instance, iron reacting with oxygen to form rust, and the fact that argon does not react with anything, are both chemical properties. Chemical properties are observed in a chemical reaction and a new substance is formed. Materials: 4 small plastic cups labeled #1 (Sugar), #2 (Corn Starch), #3 (Salt), and #4 (Baking Soda) Vinegar Candle Popsicle Sticks 100mL Beaker Plastic Spoons Watch Glasses Matches Iodine Stopwatch Investigation Instructions: 1. Physical Appearance of substance: Look at the substance. Write down your observations of its appearance, texture (you may touch them – it’s safe!) 2. Dissolving in water: Place a half of spoonful of sugar in 20mL of water (use graduated cylinder to measure). Using the stir rod, stir the substance to allow dissolving to happen. This experiment should be timed to see which substance dissolves the quickest. Wash out your beaker in the sink and get fresh water for each substance you test. Repeat the same process for the other substances. 3. Vinegar reaction: Place a half of a spoonful of sugar into 10mL of vinegar (use graduated cylinder to measure). Record observations. Rinse out your beaker in the sink and get new vinegar for each substance you subsequently test. 4. Iodine test: Place a small amount of sugar, about the size of a dime, on the watch glass. Drop 1-2 drops of iodine onto the sugar. Record observations. Repeat the same process for the other substances. 5. Flammability Put your candle in some playdough to stabilize it. Light your candle and drop a small pinch of each substance on an open flame. This allows you to see if there is a flash point for the substance where it would burn. A positive test would be flare up of the flame. DO NOT THROW OUT THE PLAYDOUGH. Data Table: _________________________________________________________ Solid Substance Physical Appearance + Water + Vinegar Date: _____________________ + Iodine + Fire Baking Soda Corn Starch Sugar Salt Mystery #___ Further Investigation Instructions: After testing all of the 4 substances to identify their physical and chemical properties you need to obtain a small sample of the mystery mixture. The mystery mixture is a combination of 2 or 3 of these solid powders you have been testing. You will run the same tests you did before for the mystery mixture and based upon the physical and chemical properties you identify you should be able to identify what substances are in the mystery mixture. Lab Conclusion: 1. What substances were in your mystery mixture? Claim: _____________________________________________________________________________. Evidence: ___________________________________________________________________________ ____________________________________________________________________________________ ___________________________________________________________________________________. Reasoning: _________________________________________________________________________ ___________________________________________________________________________________. 2. Which investigations were determining chemical properties? Claim: _____________________________________________________________________________. Evidence: ___________________________________________________________________________ ____________________________________________________________________________________ ___________________________________________________________________________________. Reasoning: _________________________________________________________________________ ___________________________________________________________________________________. Lab Conclusion: 1. What substances were in your mystery mixture? Claim: _____________________________________________________________________________. Evidence: ___________________________________________________________________________ ____________________________________________________________________________________ ___________________________________________________________________________________. Reasoning: _________________________________________________________________________ ___________________________________________________________________________________. 2. Which investigations were determining chemical properties? Claim: _____________________________________________________________________________. Evidence: ___________________________________________________________________________ ____________________________________________________________________________________ ___________________________________________________________________________________. Reasoning: _________________________________________________________________________ ___________________________________________________________________________________.