Physical & Chemical Properties Lab Worksheet
advertisement

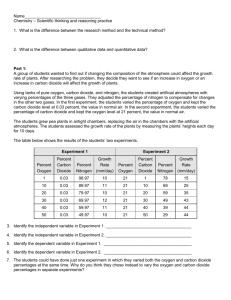
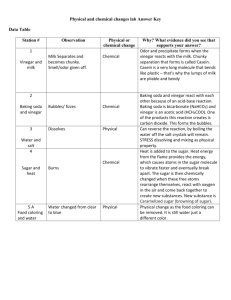
Name:__________________________ Period: 1 2 3 4 5 6 7 Date:____________________ Physical and Chemical Properties Lab Background Information: Physical properties are properties that can be observed or measured. Chemical properties are properties that have to be tested to be known. During the course of this lab you will be investigating both the physical and chemical properties of four different substances. You will also be given a mystery mixture and you will need to determine what the substances are combined together in the mystery mixture. Materials: 4 small plastic cups labeled #1 (Sugar), #2 (Corn Starch), #3 (Salt, and #4 (Baking Soda) Microscope Microscope Slides Vinegar 100mL Beaker Matches Candle Plastic Spoons Iodine Popsicle Sticks Watch Glasses Stopwatch Lab Station Instructions: 1. Shape and size of particles: On the glass microscope slide sprinkle a small amount of sugar and look at it under the microscope. Use the 4x and 10x objective lens to view the sugar. Repeat the same process for the corn starch, salt, and baking soda. 2. Dissolving in water: Place a half of spoonful of sugar in 30mL of water. Stir the substance to allow dissolving to happen. This experiment should be timed to see which substance dissolves the quickest. Wash out your beaker and get fresh water for each substance you test. Repeat the same process for the other substances. 3. Vinegar reaction: Place a half of a spoonful of sugar into 10mL of vinegar. Record on your data chart whether or not the sugar reacted with the vinegar. Clean out your beaker and get new vinegar for each substance you subsequently test. 4. Iodine test: Place a small amount of sugar, about the size of a dime, on the watch glass. Drop 2-3 drops of iodine onto the sugar. Repeat the same process for the other substances. 5. Flammability Light your candle and drop a small pinch of each substance on an open flame. This allows you to see if there is a flash point for the substance where it would burn. A positive test would be flare up of the flame. Solid Substance Baking Soda Corn Starch Sugar Salt Mystery #______ Water Fire Vinegar Iodine Crystal Shape Further Lab Instructions: After testing all of the 4 substances to identify their physical and chemical properties you need to obtain a small sample of the mystery mixture. The mystery mixture is a combination of 2 or 3 of these solid powders you have been testing. You will run the same tests you did before for the mystery mixture and based upon the physical and chemical properties you identify you should be able to identify what substances are in the mystery mixture. Lab Questions: 1. Which mystery mixture did you experiment with (#1 or #2) 2. What substances make up the mystery mixture? 3. As a group write a conclusion on this experiment? Discuss what your unknown mixture was and what physical and chemical properties helped you determine its identity. You should write at least one to two paragraphs explaining your results? 4. What physical properties did we examine in this lab? 5. What chemical properties did we test in this lab?