ISHARJYOT DEGREE COLLEGE,PEHOWA
VAPOUR PRESSURE AND PROPERTIES OF LIQUID STATE
GUIDED BY
PRESENTED BY
MANISH KAUR
MS.AMANPREET
Submitted for the partial
fulfilment
of
B.S.C
In
Non-Medical
2022-23
1
CONTENT
Vapour pressure
Cooling caused by evaporation
Factors affecting vapour pressure
Boiling point
Heat of vaporisation
Relationship between boiling point and critical temperature
Relationship between boiling point and heat of vaporisation
Measurement of vapour pressure
Structure of liquids
Properties of liquid state
Vapour pressure and properties of liquid state
2
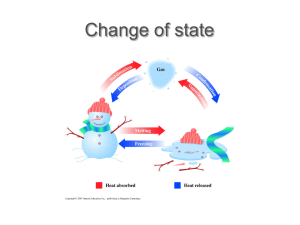
VAPOUR PRESSURE
Cooling caused by evaporation
When a liquid evaporates,
the more energetic molecules leave
the liquid .
As a result, the average kinetic energy
of the
remaining liquid decreases and hence
the
temperature falls.
Factors affecting vapour pressure
Nature of the liquid
If the intermolecular forces of attraction
in the liquid are weak,
the molecules can easily Leave
the liquid and come into vapour phase
and hence the vapour pressure is higher.
For example,
the vapour pressure of either,
acetone, benzene etc.
is higher than that of water at the same
temperature.
Effect of temperature
As the temperature of a
liquid is increased, the number of molecules
with
Higher kinetic energy increases.
Hence the vapour pressure of the liquid
increases.
Boiling point
The vapour pressure of a liquid increases as the temperature is increased.
The temperature at which the vapour pressure of the liquid becomes equal to the external
pressure is called the boiling point of the liquid.
When the external pressure is normal atmospheric pressure ,
the boiling point is called normal boiling point. For example, normal boiling point of
water is 100 degree calcius.
Obviously, if the external pressure is higher , more heat will be
required to make the vapour pressure equal to the external pressure and
hence higher will be the boiling point. Similarly, if the external pressure is decreased,
the boiling point is lowered.
This is the reason that a liquid boils at a lower temperature on
the top of a mountain ( where pressure is low ) than
on the sea shore.
Heat of vaporisation
The amount of heat required to change 1 mole of the liquid into its vapour at
the boiling point is called the heat of vaporisation of the liquid.
Relationship between boiling point and critical temperature
The normal boiling points of the liquids are nearly two-third of their critical
temperatures when both are expressed on the absolute scale.
Relationship between boiling point and heat of vaporisation
Trouton observed the following generalization
about liquids which are non-associated and which
do not have too high boiling points
For liquids which are non associated
and do not have too high boiling points ,
the ratio of heat of vaporisation to the boiling point
is approximately 21.
Measurement of vapour pressure
Structure of liquids
Liquid occupy an intermediate position between
complete disorder of gases and perfectly ordered arrangement of
solids. In liquids, there are strong cohesive force, hence the
molecules are closely packed.
This give them similarity to the solid state but the molecules in
liquids have appreciable translational
energy which inhibits the long range order. Whereas in solid state,
the kinetic energy of constituent
particles is negligible because atoms or ions can only vibrate about
their mean position.
Properties of liquid state
Incompressibility
The case with which liquids flow shows that
the molecules in liquids are randomly arranged like those of a
gas. Liquids are incompressible,
which indicates the strong forces of attraction and compact
structure of liquids resembling that of a solid.
Liquids represent an intermediate state between order and
disorder.
Change in molar volume on fusion and vaporisation
When a solid melts, its molar volume increases by about 10%
whereas the increase in volume is about 100-1000 times when a liquid is
converted into vapours at its boiling point.
A small change in volume during fusion indicates that there is some
disorder when the solid melts.
But a large increase in volume when a liquid changes into vapour indicates
a large disorder from liquids to gaseous state.
It reveals that liquids change do not have perfect order as in solids but they
are not so disordered like gases.
Enthalpy of fusion and vaporisation
The amount of heart required to melt one mole of solid at the
melting point
i.e., the enthalpy of fusion, del H is found to be much lesser than
the enthalpy of vaporization , del Hv which is the heat required to
convert one mole of a liquid into vapour at its boiling points.
Entropy of fusion and entropy of vaporisation
Entropy of fusion del S i.e., the entropy change
accompanying melting
of one mole of solid at the melting point is
found
to be lesser than the entropy of vaporization del
S,
which is the change in entropy involving
vaporization
of .1 mole of liquid at its boiling point.