Daniel C Harris - Quantitative chemical analysis solution manual (2011, ) - libgen.lc
advertisement

^5
r?~->
Eighth Edition
SOLUTIONS
MANUAL FOR
Quantitative
Chemical
Analysis
Daniel C. Harris
Solutions Manual
for Harris'
Quantitative Chemical Analysis
Eighth Edition
Daniel C. Harris
Michelson Laboratory
•
W. H. Freeman and Company
New York
ISBN: 1-4292-3123-8
EAN: 978-1-4292-3123-7
© 2003,2007,2011 by W.H. Freeman and Company
All rights reserved.
Printed in the United States of America
First printing
W.H. Freeman and Company
41 Madison Avenue
New York, NY 10010
Houndmills, Basingstoke RG2I 6XS England
www.whfreeman.com
Contents
Chapter 0
The Analytical Process
1
Chapter 1
Measurements
3
Chapter 2
Tools of the Trade
12
Chapter 3
experimental Error
17
Chapter 4
Statistics
24
Chapter 5
Quality Assurance and Calibration Methods
38
Chapter 6
Chemical Equilibrium
53
Chapter 7
Activity and Systematic Treatment of Equilibrium
61
Chapter 8
Monoprotic Acid-Base Equilibria
71
Chapter 9
Polyprotic Acid-Base Equilibria
82
Chapter 10
Acid-Base Titrations
97
Chapter 11
EDTA Titrations
126
Chapter 12
Advanced Topics in Equilibrium
142
Chapter 13
Fundamentáis of Electrochemistry
175
Chapter 14
Electrodes and Potentiometry
189
Chapter 15
Redox Titrations
202
Chapter 16
Electroanalytical Techniques
217
Chapter 17
Fundamentals of Spectrophotometry
230
Chapter 18
Applications of Spectrophotometry
238
Chapter 19
Spectrophotometers
251
Chapter 20
Atomic Spectroscopy
260
Chapter 21
Mass Spectrometry
269
Chapter 22
Introduction to Analytical Separations
284
Chapter 23
Gas Chromatography
298
Chapter 24
High-Performance Liquid Chromatography
311
Chapter 25
Chromatographic Methods and Capillary Electrophoresis
327
Chapter 26
Gravimetric Analysis, Precipitation Titrations,
345
and Combustion Analysis
Chapter 27
Sample Preparation
360
CHAPTER O
THE ANALYTICAL PROCESS
Qualitative analysis finds out what is in a sample. Quantitative analysis measures
how much is in a sample.
Steps in a chemical analysis:
(1) Formulate the question: Convert a general question into a specific one that
can be answered by a chemical measurement.
(2) Select the appropriate analytical procedure.
(3) Obtain a representative sample.
(4) Sample preparation: Convert the representative sample into a sample suitable
for analysis. If necessary, concentrate the analyte and remove or mask
interfering species.
(5) Analysis: Measure the unknown concentration in replicate analyses.
(6) Produce a clear report of results, including estimates of uncertainty.
(7) Draw conclusions: Based on the analytical results, decide what actions to
take.
Masking converts an interfering species to a noninterfcring species.
A calibration curve shows the response of an analytical method as a function of
the known concentration of analyte in standard solutions. Once the calibration
curve is known, then the concentration of an unknown can be deduced from a
measured response.
(a) A homogeneous material has the same composition everywhere. In a
heterogeneous material, the composition is not the same everywhere.
(b) In a segregated heterogeneous material, the composition varies on a large
scale. There could be large patches with one composition and large patches
with another composition. The differences are segregated into different
regions. In a random heterogeneous material, the differences occur on a fine
scale. If we collect a "reasonable-size" portion, we will capture each of the
different compositions that are present.
(c) To sample a segregated heterogeneous materia!, we take representative
amounts from each of the obviously different regions. In panel b in Box 0-1,
66% of the area has composition A, 14% is B, and 20% is C. To construct a
I
Chapla
representative bulk sample, we could take 66 randomly selected samples
from region A, 14 from region B, and 20 from region C. To sample a
random heterogeneous material, we divide the material into imaginary
segments and collect random segments with the help of a table of random
numbers.
We are apparently observing interference by Mn2+ in the I' analysis by method
A. The result of the I" analysis is affected by the presence of Mn 2+ . The greater
the concentration of Mn 2+ in the mineral water, the greater is the apparent
concentration of F found by method A. Method B is not subject to the same
interference, so the concentration of F ¡s low and independent of addition of
Mn 2+ . There must be some Mn 2+ in the original mineral water, which causes
method A to give a higher result than method B even when no Mn 2+ is
deliberately added.
CHAPTER 1
MEASUREMENTS
A note from Dan: Don't worry if your numerical answers are slightly different
from those in the Solutions Manual. You or I may have rounded intermediate
results. In general, retain many extra digits for intermediate answers and save
your roundoff until the end. We'll study this process in Chapter 3.
1-1.
(a) meter (m), kilogram (kg), second (s), ampere (A), kelvin (K), mole (mol)
(b) hertz (Hz), newton (N), pascal (Pa), joule (J), watt (W)
1-2.
Abbreviations above kilo are capitalized: M (mega, 106), G (giga, 109), T (tera,
10l2),P(peta, 10,5),E(exa, lO^.Zizetta, lO^and Y (yotta, 1024).
1-3.
(a) mW
(b) pm
(c) kn
(d) uF
(e) TJ
(f) ns
(g) fg
(h) dPa
1-4.
(a) lOOfJorO.lpJ
(d) 0.1 nm or 100 pm
(b) 43.172 8 nF
(e) 21 TW
=
=
=
=
=
=
=
milliwatt
picometer
kiloohm
microfarad
terajoule
nanosecond
femtogram
decipascal
=
=
=
=
=
-
10-3watt
10-12 meter
103ohm
10"6 farad
10l2joule
10"9 second
10 15 gram
lO"1 pascal
(c) 299.79 THz or 0.299 79 PHz (f) 0.483 amol or 483 zmol
1-5.
(a) 5.4 Pg = 5.4x1015 g.
5.4 * 10" / * J ^ T
= 5.4 x l0l 2 kgofC
(b) The formula mass of C0 2 is 12.010 7 + 2(15.999 4) = 44.009 5
M x 10t2kgex 4 jffio 7 J » ? =2.0xl0'3kgCO2
lt0n
in
(c)
2.0 x 10'3 r k^C0
2x
y =2.0x 10 tonsofCO 2
v
&
'
1000 jig
2.0 x 1010 tons
= 4 tons per person
5x 10* people
3
4
Chapter I
1-6.
Table 1-4 tells us that 1 horsepower •= 745.700 W = 745.700 J/s.
100.0 horsepower • (100.0 JiojsepowéT) 745.700 J / s
= 7.457 x 1()4 j / s .
7.457 x 104 f-
£
4.184 ' cal
2.2 x l 0 6 ^ P
1-7.
(a)
x 3600^- = 6.416x10?—.
h
h
4.184
j yij^Y \* \
fid)\ 24 X R3
- = 2.0 J/(s-kg)
(120^D«ricr) 0.453 6 kg
jjpuriif
= 2.0 W/kg
Similarly, 3.4 x 103 kcal
day
3.0J/(s-kg) = 3.0 W/kg.
(b) The office worker's power output is
2.2 * 1 0
6
^
4 ,84'íí41pq = 1.1 x 1 0 2 l
s
^áf;[24Xj^3600S>
1-8.
1-9.
= i.ixi()2w
The person's power output is greater than that of the 100 W light bulb.
ßCa
5.00 x 103
I = 1 . 4 7 x 1 0 3 ^ = 1.47 x 103 W
X A
£tíf"A3600s
ljadT ¥ 1 joof
km, v 0.025 4 jú){ \2jfítf\){5
in
1 mile
280 > o f
mile
= 0.621 37 km
(a)
1000
(b)
100kfn"| 0.621 37 miles Y 3.785 4-C
miles
= 51 ][
4.64T
krfi
A gallon )
' & ™
(c) The diesel engine produces 223 g C02/km, which we will convert into g/mile:
' 2 2 3 gco 2 y
Ikrñ
kni J 0.621 37 mile J
359 mile
In 15 000 miles, C0 2 = (15 000j»fiCs)(359 g¿mrtC) = 5.38 x 106 g or 5.38 x
10 kg = 5.38 metric tons. The gasoline engine produces 266 g C0 2 /km,
Measurements
which we convert into 428 g/mile or 6.42 metric tons in 15 000 miles.
1-10.
Newton = force = mass x acceleration = kg m
•s
Joule = energy = force x distance = kg
vs2,
m = kg
Vs
J
kg
/m 2 =
Pascal = pressure = force /area = kg
2
fm
m^
m •s
1-11.
pá
0.03
À
\2
'lOOO^
<tá j v
MJ
(535 >ríf )
H )
looo p4J
lton
V
[l000¿^1000>¿
1-12.
365
¿af
year
- 6
ton
year
(a) molarity = moles of solute/ liter of solution
(b) molality = moles of solute/kilogram of solvent
(c) density = grams of substance / milliliter of substance
(d) weight percent = 100 x (mass of substance/mass of solution or mixture)
(e) volume percent = 100 x (volume of substance/volume of solution or mixture)
(f) parts per million = 106 x (grams of substance/grams of sample)
(g) parts per billion = 109 x (grams of substance/grams of sample)
(h) formal concentration = moles of formula/liter of solution
1-13.
Acetic acid (CH3CO2H) is a weak electrolyte that is partially dissociated. When
we dissolve 0.01 mol in a liter, the concentrations of CH3CO2H plus CH3CO2
add to 0.01 M. The concentration of CH3CO2H alone is less than 0.01 M.
1-14.
1-15.
32.0 g / [(22.990 + 35.453) g/mol] = 0.548 mol NaCl
0.548 mol / 0.500 L = 1.10 M
1.71
mol CH3OH
(O.IOO L^sohnion) = 0.171 mol CH3OH
6
Chapter 1
(0.171 moiCHiOTT)
1-16.
32.04g
jniiLGFFjOrT
(a) 19mPa= 19 x 10 3 Pa.
- 5.48 g
19 x l O * 3 ^ x
lbar
10 5 K
= 1.9x10-7 bar
(b) T (K) = 273.15 + *C = 273.15 - 70 = 203 K
1.9 x 10 _7 >ár
n _P_
V~ RT
^ j
molX
1-17.
1 ppm -
g solute
10 6 g s o | u t ¡ o n -
since
= 1.1 x 10-8M = 11 nM
x 203 X
! L of dilute solution ~ 103 g,
I ppm = 10"3 g solute/L ( = 10 3 g solute /10 3 g solution).
Since 10-3 g = 103 u,g, 1 ppm = 103 ng/L or 1 ug/mL.
Since 10*3 g = 1 mg, 1 ppm = 1 mg/L.
1-18.
0.2 ppb means 0.2 x 10 9 g of C20H42 per g of rainwater
g C20H42
0-2 x 10-6 g C20H42
= 0.2 x 10-6
1 000 g rainwater
L rainwater
0.2xl0"V/L
-£.
= 7 x 10-1° M
282.55//mol
1-19.
0.705
g HCIO4
(37.6 solution)
£sohrtî6n~ I
=26.5gHCI04
37.6 g solution - 26.5 g HCIO4 = 11.1g H 2 0
1-20.
(a)
1.67
g solution
1000
*£'
= 1.67 x 103 g solution
J
(b)
0.705
g HCIO4
(1.67 x 103^sc4«tl6n') = 1.18 x 103 g HCIO4
jj^c4trtîoif I
(c) (1.18 x 1 0 3 / ) / ( 1 0 0 . 4 6 / / m o l ) = 11.7 mol
« ««
1-21.
...
mol Kl
molality = k g s o l v e n t
MOwtV I _
200 g Kl
_ 200 g KJ
¿u.u wt/0 KKl
]Q00è
s o l u t i o n - goo g H 2 0
Tofindthe grams of KI in 1 kg of H2O, we set up a proportion:
7
Measurements
200 fi Ki
s^ja_
800 g H 2 0 " 1 000 g H 2 0 => x
1-22.
z:)U g R l
But 250 g KI = 1.51 mol KI, so the molality is 1.51 m.
amol
150 x IQ 15 mol/cetî
(a) Z5 x l o 4 v e s i c l e s / i ; e H . - 6*°vessicle
r"
(b) (6.0 x KT 18 jtfoí) 6.022 x 10
23 molecules
••= 3.6 x 10f> molecules
V
(c) Volume = - n ( 2 0 0 x 10- 9 m) 3 = 3.35 x 10- 20 m 3 ;
3.35 x 1Q- 2 V 3
10" V /L
10 « 10- ,8 mol .
3.35 x 10~ I7 L
80xlO"V
1-23.
=335xl0
.17L
0 30 M
1A.
4
T — £ - = 4.4 x lO" mol;
180.2//mol
4.4x1o- 4 mol
. . ,.,..
—
=
4.4
x 10"3 M;
5
3
100xlO* L
Similarly, 120 mg/100 L = 6.7 x 10' 3 M.
1-24.
(a) Mass of 1.000 L = 1 0 4 6 " ^ x 1 OOO—7- x 1.000 X = 1 046 g
Grams ofC 2 H 6 0 2 per liter = 6 . 0 6 7 ^ 1 * 6 2 . 0 7 - J - , = 376.6^
(b) 1.000 L contains 376.6 g of C 2 H 6 0 2 and 1046 - 376.6 = 669 g of H 2 0
= 0.669 kg
Molality-
1-25.
6.067 mol C 2 H 6 02
0.669 k g H 2 0
= 907
mol C2Hr>02
=
kgH20
9 0 7 w
Shredded wheat: 1.000 g contains 0.099 g protein + 0.799 g carbohydrate
0 . 0 9 9 / x 4 . 0 ^ r + 0 . 7 9 9 / x 4 . 0 y = 3.6 Cal
Doughnut: 1.000 g contains 0.046 g protein + 0.514 g carbohydrate + 0.186 g fat
0 . 0 4 6 / x 4 . 0 y + 0 . 5 1 4 / * 4 . 0 ^ y + 0 . 1 8 6 / x o . 0 ^ ¥ = 3.9 Cal
Cal
Cal
In a similar manner, we find 2.8 —~ for hamburger and 0.48 ~~~ for apple.
There are 16 ounces in 1 pound, which Table 1-4 says is equal to 453.592 37 g
8
Chapter 1
28.35
ounce
To convert Cal/g to Cal/ounce, multiply by 28.35:
Shredded Wheat
Cal/g
Cal/ounce
1-26.
3.6
102
Doughnut
Hamburger
Apple
3.9
111
2.8
79
0.48
14
Mass of water = n (225 jrf)2 (10.0 rf) [ " " T ^ 8 ] = 1.59 x 109 kg
1-6 p p m -
1.6 x 1Q-3BF~
kgH20
Mass of F~ required =
*-3
1.6x10"
gF
JcgJ^J
(I.59xl0 9 kg-H^O") = 2.5 x 1 0 6 g F .
(If wc retain three digits for the next calculation, this last number is 2.54 x
106.)
The atomic mass of F is 18.998 and the formula mass of H 2 SiF 6 is 144.09. One
mole of H2SiF6 contains 6 moles of F.
mass of F~ _ 6 x 18.998
massofH 2 SiF 6 '' 144.09
1-27.
2.54 * 10 6 gF
xgH 2 SiF 6 ^
x
~
3 2
* l0
g H SlF
2 <>
(a) PV = nRT
(
(1.000 bar)(5.24x 10-* L) = n 0.08314 L • bar Ï(298.15/Q
mol-K
,
••V
7
n = 2.11 x lo? mol _> 2 .i i x 10" M
(b) Ar: 0.934% means 0.00934 L of Ar per L of air
L ' bar
(1.000 bai-)(0.00934 L) = n\ 0.083 14
(298.15 K)
mol * VLj
=> n ="S.11x lO"4 mol => 3.77 x 10'4 M
Kr: 1.14 ppm => 1.14 uL Kr per Lof air => 4.60 x 10"8M
Xc: 87ppb=> 87nLXeperLofair => 3 . 5 x l 0 ' 9 M
1-28.
2.00 X * 0.050 0 ^ P * 61 -83-J-j, = 6.18 g in a 2 L volumetric flask
1-29.
Weigh out 2 x 0.0500 mol = 0.100 mol = 6.18 g B(OH)3 and dissolve in 2.00
kg H 2 0.
9
Measurements
1-30.
M con • Kcon = Mdii * Vá\\
V s O ^ V o o X ) = i o . 2 5 ^ 1 Vm =* Kdfl = 3.2 L
1-31.
We need 1.00 X
4,0 EÍslaOTÍ
£-^
x
0 . 1 0 2 ^ = 0.10 mol NaOH = 4.0gNaOH
= 8.0 g solution
g solution
1-32.
Mdii
. rt.OOMPi CC£ ¥
(a) ^con - KdHl M ^ ; = 1 000 mL [j^^)
= 55.6 mL
(b) One liter of 98.0% H2SO4 contains (18.0 ^ioí)(98.079g/jHoí) - 1.77 x 103
g of H 2 S04. Since the solution contains 98.0 wt% H2SO4, and the mass of
H2SO4 per mL is 1.77 g, the mass of solution per milliliter (the density) is
1.77^gJi2SCM/mL
0.980 ^ H ^ S O T / g solution
1-33.
= 1.80gsolution/mL
2.00Lof0.169MNaOH = 0.338 mol NaOH = 13.5 g NaOH
g solution
density = m L s o l u t i o n1
13.5¿NaOrf
152
(16.7 mL solution) 0 . 5 3 4 * ^
0e solution
1-34.
FM of Ba(N03)2 - 261.34
_g_
mL
/
4.35 g of solid with 23.2 wt% Ba(N03)2 contains
(0.232)(4.35 g) - 1.01gBa(NO3h
a2+
.
(uigfrpiosfi)
(261.34 zßztfiOih
.
3 8 6 x 10.3mol
I mol)
mol H2SO4 = mol Ba 2+ = 3.86 x lO"3 mol
(3.86 x IQ-3 mol)
,„
.
volume of H2SO4 =
- 1 -29 mL
(3 0 0 m o | / L )
1-35.
25.0 mL of 0.023 6 M Th 4+ contains
(0.025 0 LX0.023 6 M) = 5.90 x 10-4 mol Th 4+
mol HF required for stoichiometric reaction = 4 x mol Th 4+ = 2.36 x 10"3 mol
50% excess = 1.50(2.36 x 10-3 mol) = 3.54 x 10-3 mol HF
10
Chapter I
Required mass of pure HF = (3.54 x 10'3 mol)(20.01 g/mol) = 0.070 8 g
Mass of 0.491 wt% HF solution =
(0-070 8 ^ H f )
(0.004 91 ßJHf/g solution)
=
5
1-36.
Concentrations of reagents used in an analysis are determined cither by weighing
out supposedly pure primary standards or by reaction with such standards. If the
standards arc not pure, none of the concentrations will be correct.
1 -37.
The equivalence point occurs when the exact stoichiometric quantities of reagents
have been mixed. The end point, which comes near the equivalence point, is
marked by a sudden change in a physical property brought about by the
disappearance of a rcactant or appearance of a product.
1-38.
In a blank titration, the quantity of titrant required to reach the end point in the
absence of analyte is measured. By subtracting this quantity from the amount of
titrant needed in the presence of analyte, we reduce the systematic error.
1-39.
In a direct titration, titrant reacts directly with analyte. In a back titration, a
known excess of reagent that reacts with analyte is used. The excess is then
measured with a second titrant.
1-40.
Primary standards are purer than reagent-grade chemicals. The assay of a
primary standard must be very close to the nominal value (such as 99.95100.05%), whereas the assay on a reagent chemical might be only 99%. Primary
standards must have very long shelf lives.
1-41.
Since a relatively large amount of acid might be required to dissolve a small
amount of sample, we cannot tolerate even modest amounts of impurities in the
acid for trace analysis. Otherwise, the quantity of impurity could be greater than
quantity of analyte in the sample.
1-42.
40.0 mL of 0.0400 M Hg 2 (N0 3 ) 2 = 1.60 mmol of Hg224 , which will require 3.20
mmol of KI. This is contained in volume = 0 ,
1-43.
m
—r,—¡- = 32.0 mL.
108.0 mL of 0.165 0 M oxalic acid - 17.82 mmol, which requires
2 mol Mn0 4 I
,5 mol H2C2O4J( ] 7 ' 8 2 m o 1 H 2 C 2 ° 4 ) =7.128 mmol of Mn0 4
M
Measurements
7.128 mmol / (0.165 0 mmol/mL) = 43.20 mL of KMn0 4 .
Another way to see this is to note that the reagents are both 0.165 0 M. Therefore,
2
volume of Mn0 4 = f(volume of oxalic acid).
For second question, volume of oxalic acid = ^volume of Mn0 4 ) = 270.0 mL.
1-44.
1.69mgofNH3 = 0.0992 mmol of NH3. This will react with | (0.099 2) 0.149 mmol of OBr". The molarity of OBr" is 0.149 mmol/1.00 mL = 0.149 M.
A I'll 1
1-45.
mol sulfamic acid = 97 094 o/mo\
molarity of NaOH =
1-46.
3.43Ó9 mmol
34 26 m L
=
^-4369
mm
°l
= 0.1003 M
HCl added to powder = (10.00 mL)(1.396 M)= 13.96 mmol
NaOH required = (39.96 mL)(0.1004 M) = 4.012 mmol
HCl consumed by carbonate = 13.96 - 4.012 = 9.948 mmol
mol CaC03 - \ mol HCl consumed = 4.974 mmol = 0.497g g CaC03
0.497s g CaC0 3
wt%CaC0 3 • 0.541 3 g limestone * l 0 ° =
920wt%
CHAPTER2
TOOLS OF THE TRADE
The primary rule is to familiarize yourself with the hazards of what you are about
to do and not to do something you consider to be dangerous.
Dichromate (Cr202") is soluble in water and contains carcinogenic Cr(Vl).
Reducing Cr(VI) to Cr(III) decreases the toxicity of the metal. Converting
aqueous Cr(III) to solid Cr(OH)3 decreases the solubility of the metal and
therefore decreases its ability to be spread by water. Evaporation produces the
minimum volume of waste.
The upper "0" means that the reagent has nofirehazard. The right hand "0"
indicates that the reagent is stable. The "3" tells us that the reagent is corrosive
or toxic and we should avoid skin contact or inhalation.
The lab notebook must: (1) state what was done; (2) state what was observed; and
(3) be understandable to a stranger.
See Section 2.3.
The buoyancy correction is 1 when the substance being weighed has the same
density as the weights used to calibrate the balance.
(14.82g)(l- jjg-gg J
m
-
=
( 0.0012g/mL\
V ~~ 0.626 g/mL J
,4 85
" «
The smallest correction will be for Pb02, whose density is closest to 8.0 g/mL.
The largest correction will be for the least dense substance, lithium.
.....
(
4.236 6 g [\m
0.001 2 g/mL^
8Qg/%L
J
= 4239 1
( 0.001 2 K/mL^
S
[} 1.636 g/mL )
Without correcting for buoyancy, we would think the mass of primary standard is
less than the actual mass and we would think the molarity of base reacting with
the standard is also less than the actual molarity. The percentage error would be
true mass - measured mass . „
4.239 1 - 4.236 6
l0
4^391
* , 0 ° = <>•<*%.
iríais
* ° =
-
12
13
Tools of the Trade
2-11.
(a) One mol of He (= 4.003 g) occupies a volume of
(IjHoí)í0.08314—JT/
V=
|(293.15X)
\mt5i-X
nRT
- 24.37 L
Density = 4.003 g / 24.37 L =- 0.164g/L « 0.000 164 g/mL
0.000164 £¿mfT
8.0 jj/mL" y
(0.823 g)
(b)m
2-12.
f
_
0.000164 ^g/HítT
0.97jj¿HifT
0.823 g
(a) (0.42) (2 330 Pa) = 979 Pa
(b) Air density =
(0.003485K94000)-(0.OO1318X979)
293.15
'
(c) mass = 1.0000 g
o.ooi
=
^
^
= QQQl
]
^
\^C
0.0011 £¿mlf
11.00 gxiC J
= 1.0010 g
2-13.
mb = ma^
= (100.0000 g) f6 370 030 ^ 2 - 99.999 1 g
2-14.
TD means "to deliver" and TC means "to contain."
2-15.
Dissolve (0.250 0 L)(0.150 0 mol/L) - 0.037 50 mol of K 2 S0 4 (= 6.535 g, FM
174.26 g/mol) in less than 250 mL of water in a 250-mL volumetric flask. Add
more water and mix. Dilute to the 250.0 mL mark and invert the flask many
times for complete mixing.
2-16.
The plastic flask is needed for trace analysis of analytes at ppb levels that might
be lost by adsorption on the glass surface.
2-17.
(a) With a suction device, suck liquid up past the 5.00 mL mark. Discard one or
two pipet volumes of liquid to rinse the pipet. Take up a third volume past
the calibration mark and quickly replace the bulb with your index finger.
(Alternatively, use an automatic suction device that remains attached to the
14
Chapter 2
pipet.) Wipe excess liquid off the outside of the pipet with a clean tissue.
Touch the tip of the pipet to the side of a beaker and drain liquid until the
bottom of the meniscus reaches the center of the mark. Transfer the pipet to a
receiving vessel and drain it by gravity while holding the tip against the wall.
After draining stops, hold the pipet to the wall for a few more seconds to
complete draining. Do not blow out the last drop. The pipet should be nearly
vertical at the end of delivery.
(b) Transfer pipet.
2-18.
(a) Adjust the knob for 50.0 uL. Place a fresh tip tightly on the barrel. Depress
the plunger to the first stop, corresponding to 50.0 pL. Hold the pipet
vertically, dip it 3-5 mm into reagent solution, and slowly release the plunger
to suck up liquid. Leave the tip in the liquid for a few more seconds.
Withdraw the pipet vertically. Take up and discard three squirts of reagent to
clean and wet the tip and fill it with vapor. To dispense liquid, touch the tip
to the wall of the receiver and gently depress the plunger to the first stop.
After a few seconds, depress the plunger further to squirt out the last liquid,
(b) The procedure in (a) is called forward mode. For a foaming liquid, use
reverse mode. Depress the plunger beyond the 50.0 (iL stop and take in
more than 50.0 uL. To deliver 50.0 p.L, depress the plunger to the first stop
and not beyond.
2-19.
The trap prevents liquid fíltrate from being sucked into the vacuum system. The
watchglass keeps dust out of the sample.
2-20.
Phosphorus pentox i de
2-21.
20.2144 g - 10.2634 g = 9.951 0 g. Column 3 of Table 2-7 tells us that the true
volume is (9.951 0 g)(l .002 9 mL/g) - 9.979 9 mL.
, ,,
2-22.
0.999102 6
Expansion = 0.997 047 9
B
=
'
002
0°° 8 ~ °-2%- Densities were taken from Table
2-7. The 0.500 0 M solution at 25° would be (0.500 0 M)/( 1.002) = 0.499 0 M.
2-23.
Using column 2 of Table 2-7, mass in vacuum =
(50.037jntTX0.998 207 1 g/^mtT) = 49.947 g.
Using column 3, mass in air =
I
T
•
1
1
=--^- = 49.892 e.
1.0029>ffL7g
*
5
0
.
0
3
7
p
¿
t
A
t
M
15
Tools of the Trade
2-24.
When the solution is cooled to 20°C, the concentration will be higher than the
-..„.
*
*• density at 20°C _,
_
,.
concentration at 24 C by a factor of density at 24°C- Therefore, the concentration
needed at 24* will be lower than the concentration at 20°C.
'0.997 299 5^/imO
0.999 1 M
Desired concentration at 24'C = (1.000 M)
0.998 2071^/rnL^
(using the quotient of densities from Table 2-7). The true mass of KNO3
needed is (o.5000X) 0 . 9 9 9 1 ^ -
101.103-jM = 50.506 g.
(50.506 g ) [ l - 2 1 0 9 J m L J
m
' =
2-25.
(
0.001 2 R^mñ
\}~
8.0 g/mL )
=
50
-484g
(a) Fraction within specifications = e"^" 2)"m. If tm = 2 yr and / = 2 yr, then
fraction within specifications = e"™n 2 ^ = e"ln 2 = lA.
(b) Fraction within specifications = 0.95 • e ^ n 2 ^ 3"
ln(0.95) • -/(In 2)/2 => t = -2 ln(0.95)/ln 2 = 0.148 yr = 54 days * 8 weeks
To solve for /, take the natural logarithm of both sides:
2-26.
Al extracted from glass = (0.200 L)(5.2 x 10"6 M) = 1.04 x 10"6 mol
mass of Al = (1.04 x 10' 6 mol)(26.98 g/mol) = 28.1 pg
This much Al was extracted from 0.50 g of glass, so
wt% Al extracted = 100 x
Fraction of Al extracted -
6
28 1 x 10"
ft ß
050g
- 0.005 6 2 wt%
0.005 6 2 wt%
= 0.007 0 (or 0.70% of the Al)
Q 8 Q wt o /o
16
2-27.
Chapter 2
I
Graph of van Deemter Equation
I
I
van Deemter Equation
Flow rate Plate height
(mm)
Constants (mUmin)
A=
4
8.194
1.65
6.092
6
B=
8
5.064
10
25.8
4.466
C=
20
3.412
30
0.0236
3.218
40
3.239
50
3.346
60
3.496
70
3.671
80
3.861
90
4.061
100
4.268
Formula;
t^N> —
vnw^r.Jt.TVv'OÍDJT^ •M>
IU D3
9
8
?
7
1
A
lu
3
£
2
' S 6
1O) 5J
1
0
_A
0
—
i_,—
20
40
.
—,_,—
60
80
Flow rate (mUmin)
I
I
I
100
CHAPTER 3
EXPERIMENTAL ERROR
3-1.
(a) 5
(b) 4
(c) 3
3-2.
(a) 1.237
(b) 1.238
(c) 0.135
3-3.
(a) 0.217
(b) 0.216
(c) 0.217
3-4.
(b) 1.18 (3 significant figures)
3-5.
(a) 3.71
(b) 10.7
(d) 2.1
(e) 2.00
(c) 0.71 (2 significant figures)
(c) 4.0 x io> (d) 2.85 x 10-6
4
(c) 12.6251(f) 6.0 x lO" (g) 242
3-6.
(a) BaF2 = 137.327 + 2(18.998 403 2) = 175.324 because the atomic mass of Ba
has only 3 decimal places,
(b) C 6 H40 4 = 6(12.0107)+ 4(1.00794)+ 4(15.9994) =140.0936
(The fourth-decimal place in the atomic mass of C has an uncertainly of ± 8
and the fourth-decimal place of O has an uncertainty of ± 3. These uncertainties are large enough to make the fourth-decimal place in molecular mass
of C6H4O4 insignificant. Therefore, another good answer is 140.094.)
3-7.
(a) 12.3
(e) 3.04 x 10-10
(b) 75.5
(0 11.9
(c) 5.520 x 1<>3
(g) 4.600
(d) 3.04
(h) 4.9 x 10-7
3-9.
All measurements have some uncertainty, so there is no way to know the true
value.
3-10.
Systematic error is always above or always below the "true value" if you make
replicate measurements. In principle, you can find the source of this error and
eliminate it in a better experiment so the measured mean equals the true mean.
Random error is equally likely to be positive or negative and cannot be
eliminated. Random error can be reduced in a better experiment.
3-11.
The apparent mass of product is systematically low because the initial mass of
the (crucible plus moisture) is higher than the true mass of the crucible. The error
is systematic. There is also always some random error superimposed on the
systematic error.
3-12.
(a) 25.031 mL is a systematic error. The pipet always delivers more than it is
17
IN
Chapter 3
rated for. The number ± 0.009 is the random error in the volume delivered.
The volume fluctuates around 25.031 by ±0.009 mL.
(b) The numbers 1.98 and 2,03 mL are systematic errors. The buret delivers too
little between 0 and 2 mL and too much between 2 and 4 mL. The observed
variations ±0.01 and ±0.02 are random errors.
(c) The difference between 1.9839 and 1.9900 g is random error. The mass will
probably be different the next time I try the same procedure.
(d) Differences in peak area arc random error based on inconsistent injection
volume, inconsistent detector response, and probably other small variations
in the condition of the instrument from run to run.
3-13.
(a) Carmen
(b) Cynthia
(c) Chastity
(d) Cheryl
3-14.
3.124 (±0.005), 3.124 (±0.2%). It would also be reasonable to keep an
additional digit: 3.1236 (±0.0052), 3.1236(±0.17%)
3-15.
(a)
6.2 (±0.2)
-4.1 (±0.1)
2.1 ± e e2 = 0.22 + 0.1 2 => e = 0.224 Answer: 2.1 ± 0.2 (or 2.1 ± 11%)
(b)
9.43 (±0.05)
x 0.016 f±0.00n
0.150 88 (± %e)
9.43 (±0.53%)
=>
=>
x 0.016 (:i 6.25%)
%e = 6.272
Vœ2 = 0.532 + 6.252
Relative uncertainty = 6.27%; Absolute uncertainty • 0.150 88 x 0.062 7
Answer: 0.151 ±0.009
= 0.00946;
(or 0.151 ±6%)
(c) The first term in brackets is the same as part (a), so we can rewrite the
problemas 2.1 (±0.224) + 9.43(±0.05) = 2.1 (±10.7%) + 9.43 (±0.53%)
%e m <N/10.72 + 0 . 5 3 2 -
10.7%
Absolute uncertainty = 0.107 x 0.223 = 0.023 9
Answer: 0.223 * O.O24 (±11%)
(d) The term in brackets is
6.2 (±0.2) x 10-3
+ 4.1 (±0.1) x 10-3
e = V 0 2 2 + 0.1 2 => e = 0.224
10.3 (±0.224) * lO 3 = 10.3 x 10-3 (±2.17%)
9.43 (±0.53%) x 0.0103 (±2.17%) = 0.097 13 ± 2.23% = 0.097 13 ± 0.002 17
Answer: 0.097i ± 0.0022 (± 2-2%)
19
Experimental Error
3-16.
(a) Uncertainty = %/0.032 + 0.022 + 0.062 = 0.07
Answer: 10.18 (±0.07) (±0.7%)
(b) 91.3 (±1.0) x 40.3 (±0.2)/21.1 (±0.2)
= 91.3 (± 1.10%) x 40.3 (±0.50%)/21.1 (±0.95%)
% uncertainty = >/l.l0 2 + 0.502 + 0.952 = 1.54%
Answer: 174 (±3) (±2%)
(c) [4.97 (±0.05)-1.86 (±0.01 )]/21.1 (±0.2)
= [3.11 (±0.0510)]/21.1 (±0.2) = [3.11 (±1.64%)]/21.1 (±0.95%)
= 0.147 (±1.90%) = 0.147 (±0.003) (±2%)
(d)
2.0164 (±0.0008)
1.233 (±0.002)
+ 4.61 (±0.01)
7.8594 VíO.OOO 8)2 + (0.002)2 + (0.01)2 = 0.0102
Answer: 7.86 (±0.01 )(±0.1 %)
(e)
2016.4 (±0.8)
+ 123.3 (±0.2)
+ 46.1 (±0.1)
2185.8 V(0.8)2 + (0.2)2 + (0.1)2 = 0.8
Answer: 2 185.8 (±0.8) (±0.04%)
(f) Fory = xa,%ey = a%ex
* = 3.14 ±0.05 => %ex = (0.05/ 3.14) x 100 = 1.592%
%ey = j (1.592%) = 0.531%
Answer: 1.4643 ± 0.0078 (±0.53%)
(g) For y = log x, ey = 0.434 29 -f
* = 3.14 ±0.05 ^ ey = 0.434 29 (f^jfj = 0.006915
Answer: 0.4969 ± O.OO69 (± 1.39%)
3-17.
(a) y = * 1/2 ^ %ey = i ( 1 0 0 * f f t f s ) = 0.017 5%
(1.75 x 10-4)^3.1415 = 3.1 x lO-4
(b) y = log* => ey = 0.43429(f^}j)
Answer: 0.497 14± 0.000 15
Answer: 1.77243±0.00031
=
1 5 2 X 10 4
"
20
Chapter 3
(c) y = antilogy = I O1 => ey=y x 2.302 6 e*
= (103.1415)(2.3026)(0.001 !) = 35, A n s w e r : 1385 2 ±0.003 5 x 103
0.001 1
(d) y = In x => ey = 3 l 4 [ 5 = 3.5 x \Q-4
(e) Numerator of log term: y = xm
0.3225 ± 2.88%
0.0511 + 0.0009
=
Answer: 1.144 7 0 ± 0.000 3 5
=> ey = 2\fi~\Ö4x
100
J
=
2.88%
0.3225 ± 2.88%
0.0511 ± 1.76%
= 6.311+3.375% = 6.311 ±0.213
For.y= \ogx,ey
= 0.434 2 9 ^ = 0.434 29 (f^fyj) = 0.015
Answer: O.80n±0.015
3-18.
(a) Standard uncertainty in atomic mass is equal to the uncertainty listed in the
periodic tabic divided by -\ß because atomic mass has a rectangular
distribution of values.
Na = 22.989 769 28 ± 0.000 000 02/^3 g/mol
CI = 35.453
±0.002A/3 g/mol
58.442770
yj[(2 * 10"8)2]/3 + [(2 * 10-3)2]/3 = 1.2 x io-3
58.443 ±0.0012 g/mol
(b) molaritv = 3 2 ! _ T2.634 (±0.002)g1 / [58.443 (±0.001?)g/moll
C,D; moiaruy
0.10000 (±0.00008) L
L
2.634 (±0.076%) / [58.443 (±0.002 1%)
0.100 00 (0.08%)
relative error = V(0.076%)2 + (0.002 1%)2 + (0.08%)2 = 0.11%
molarity = 0.4507 (±0.000 5) M
m
3-19.
m=
ÍLá
«4
>
r i m ^ + n n o n ^ 1(1 - 0-00» 2(±0.0001)g/mL
y
[1.0346(±0.0002)g]^l
J>
8Q (±05)R/t;[
m
'
0.001
'012(±0.0001)g/n
2(±0.0001) g/mL
0.997 299 5 g/mL
21
Experimental Error
f 0.001 2 (±8.33%)
[1.0346 (±0.0193%)] [ l - 8 , o ( : 4 2 5 % ) ;
m
m
-
-
1
0.001 2 (±8.33%)
"0.997 299 5 (±0%)
n.0346(±0.019 3%)ïïl 0.000 150 (±10.4%)]
[1 - 0.001 203 (±8.33%)]
fi.0346 (±0.0193%)] [I -0.000 150 (±0.0000156)1
[ l - 0.001 203 (±0.000 100)]
"'
m
-
[ 1.034 6 (±0.019 3%)1 fO.999 850 0 (±0.000 015 6)]
[0.998 797 (±0.000 100)]
m
-
f 1.034 6 (dfcO.Q 19 3%)1 r0.999 850 0 (+0.001 56%)]
[0.998 797 (±0.010 0%)]
m = 1.035 7 (±0.021 8%) = 1.035 7 (±0.000 2) g
3-20.
0.2774 ±0.0018 g
m o l F e 2 0 3 - 159.688 g/mol
0-2774
159.688
±
O.OOlg
159.688
= 1.7371 ±0.0113 mmol Fe203;
mass of Fe = 2[1.737i (±0.0113) x 10"3 mol][55.845 g/mol] = 0.19402 + 0.00126 g
mass of Fe per tablet = (0.194o2 + 0.00126g)/12 = 16.168 ±0.105 mg
= 16.2 + 0.1 mg
3-21.
mol H + = 2 x m o l Na 2 C0 3
0.967 4 (±0.000 9) g
molNa2C0 3 = 105.988 4( \ 0.000 7) g/mol
=l
0.9674 (±0.093%) g
105.988 (±0.00066%) g/mol
= 0.009 127 4 (±0.093%) mol
molH + = 2(0.009 1274 (±0.093%)) - 0.018255 (±0.093%) mol
(Relative error is not affected by the multiplication by 2 because mol H +
and uncertainty in mol H+ are both multiplied by 2.)
0.018 255 (±0.093%) mol
0.018 255 (±0.093%) mol
molarity of HCl - 0.027 35 (±0.00004) L ~ 0.027 35 (±0.146%) L
= 0.66746 (±0.173%) = 0.66746 (±0.001 155)
= 0.667 + 0.001 M
3-22.
To find the uncertainty in c 0 3 , we use the function y = Xa in Table 3-1,
where x = c 0 and a = 3. The uncertainty in c 0 3 is
%ey = a%ex
= 3x °
^
^
^
100 = 1.823 * 10-5%
So Co3 - (5.431 020 36 x 10"8 cm) 3 = 1.601 932 796 0 x 10"22 cm3 with a
22
Chapter 3
relative uncertainty of 1.823 * 10-5%. We retain extra digits for now and round
off at the end of the calculations. (If your calculator cannot hold as many digits
as wc need for this arithmetic, you can do the math with a spreadsheet set to
display 10 decimal places.)
The value of Avogadro's number is computed as follows:
*
=
A SS
wsi = .
28.085 384 2 g/mol
3
=
(/*o )/8 " (2.329 031 9 g/cm3 * 1.601 932 79 60 * 10 2 2 cm3)/8
- 6.022 136 936 l x 1023 mol"!
The relative uncertainty in Avogadro's number is found from the relative
uncertainties in msi, p, and c 0 3 . (There is no uncertainty in the number 8
atoms/unit cell.)
percent uncertainty
percent uncertainty
percent uncertainty
percent uncertainty
in msi = 100 (0.000 003 5/28.085 384 2) = 1.246 x 10-5%
in p - 100(0.000 001 8/2.329 031 9) = 7.729 x 10-5%
in c 0 3 = 1.823 x 10"5% (calculated before)
in NA = sj%em^ + %e¿ + (%eCo3)2 =
= V0.246x 10-5)2 +(7.729 x 10-5)2 + (1.823 x 10-5)2 =
8 0 3 8 x I 0 -5o / o
The absolute uncertainty in /VA is (8.038 x 10"5%)(6.022 136 936 1 x 1023)/100
= 0.000 004 841 x 1023. Now we will round off /VA to the second digit of its
uncertainty to express it in a manner consistent with the other data in this
problem:
NA = 6.022 136 9 (±0.000 004 8) x 1<)23 or 6.022 136 9 (48) x 1023
3-23.
C: 12.010 7 +0.000 8/\/3; H: 1.007 94 + 0.00007/^3
O: 15.9994+0.000 3/V3; N: 14.006 7 + 0.0002/^3
+9C: 9(12.010 7 +0.000 46)
=
+9H: 9(1.00794 +0.000 040)
=
+60: 6(15.9994 ±0.0001 7 )
+3N: 3(14.0067 ±0.0001 2 )
C9H9O6N3:
=
=
108.096 3 + 0.004 2
9.07146 + 0.000 36
95.996 4 + 0.0010
42.020 1+0.000 35
255.184 26 ± ?
Uncertainty = A/O.004 2 2 + 0.000 362 + 0.001 0 2 + 0.000 35 2 = 0.004
Answer: 255.184 + 0.004
Experimental Error
3-24.
Relative uncertainties:
Large volume: 0.000 5 L/5.013 82 L = 0.010%
Small volume: 0.000 9 mL/3.793 0 mL = 0.024%
Pressure: 0.03 mm/400 mm = 0.008%
Temperature: 0.03 K/300 K = 0.01%
The largest uncertainty is in the volume of the small vessel = 0.024%.
Uncertainty in C0 2 * 0.024% of 400 ppm = 0.000 24 x 400 ppm = 0.1 ppm.
23
CHAPTER 4
STATISTICS
4-1.
The smaller the standard deviation, the greater the precision. There is no
necessary relationship between standard deviation and accuracy. The statistics
that we do in this chapter pertains to precision, not accuracy.
4-2.
(a) //+0- corresponds to z = - I toz = +1. The area from z = Otoz = +1 is
0.341 3. The area from z = 0 to z - -1 is also 0.341 3.
Total area (= fraction of population) from z = - I to z = +1 = 0.682 6.
(b) z = - 2 t o z = +2 => area = 2 x 0.477 3 - 0.9546
(c) z = 0to z = +l => area = 0.341 3
(d) z = 0 t o z = 0.5 => area = 0.191 5
(e) Area from z = -I to z = 0 is 0.341 3. Area from z = -0.5 to z = 0 is 0.191 5.
Area from z = - l to z =-0.5 is 0.341 3-0.1915 =0.1498.
4-3.
(a) Mean = g( 1.526 60 + 1.529 74 + 1.525 92 + 1.527 31 + 1.528 94 +
1.528 04 + 1.526 85 + 1.527 93) = 1.527 67
(b)
4-4.
Standard deviation =
. /(I.52660-1.52767) 2 +- + (1.527 93 - 1.527 67)2
\j
8-1
=
°00126
(c)
Variance = (0.001 26) 2 = 1.59 x \o-6
(d)
Significant figures: x ± s = 1.5277 ± 0.0013 or 1.528 ± 0.001.
(a) 1005.3 hours corresponds to z = (1005.3 -845.2)/94.2 = 1.700.
In Table 4-1, the area from the mean to z = 1.700 is 0.455 4. The area above
z = 1.700 is therefore 0.5 - 0.455 4 = 0.044 6.
(b) 798.1 corresponds to z = (798.1 -845.2)/94.2 = -0.500.
The area from the mean to z = -0.500 is the same as the area from the mean to
z = +0.500, which is 0.191 5 in Table 4-1.
901.7 corresponds to z • (901.7-845.2)/94.2 = 0.600.
The area from the mean to z = 0.600 is 0.225 8 in Table 4-1.
The area between 798.1 and 901.7 is the sum of the two areas:
0.191 5 + 0.225 8 = 0.417 3
(c) The following spreadsheet shows that the area from -oo to 800 h is 0.315 7
24
25
Statistics
and the area from -co to 900 h is 0.719 6. Therefore, the area from 800 to
900 h is 0.719 6-0.315 7 = 0.404 0.
B
C
A
Std dev =
1 Mean =
94.2
845.2
2
3
0.3157
4 Area from - » to 800 =
0.7196
5 Area from -y- to 900 =
0.4040
6 Area from 800 to 900
7
I
8 C4 = NORMDIST(800,$A$2,$B$2rTRUE)
9 C5 = NORMDIST(900.$A$2,$B$2,TRUE)
10 C6 = C5-C4 [
4-5.
(a) Half the people with tumors have K < 0.92 and would not be identified by the
test. The false negative rate is 50%.
(b) The false positive rate is the fraction of healthy people with K > 0.92. To use
Table 4-1, we need to convert JC = 0.92 to a z value defined as
s-w
z
=
s
=
0-92-0.75
0.07
...
=
2 M
In Table 4-1, area from mean (z = 0) to z = 2.4 is 0.491 8. Area from mean to
z = 2.5 is 0.493 8. We estimate that area from mean to z = 2.43 is a little
greater than 0.492. Area above z = 2.43 is therefore 0.5 - 0.492 = 0.008.
That is, 0.8% of healthy people will have a false positive indication of cancer.
In the following spreadsheet, cell E5 computes the area below K - 0.92 with
the formula NORMDIST(0.92, $B$4,$B$5,Truc), where B4 contains K and
B5 contains the standard deviation. The area below 0.92 is found in cell E5
to be 0.992 4. The area above K = 0.92 is therefore I - 0.002 4 = 0.007 6.
G
H
B
|
C
D | E
F
A
1 Gaussian c ¡stribution for phase partitioning of plasma proteins
2
Area below cutoff
For healthy people,
3 Healthy pa ¡ents
for people with tumors
area
below
0.92
=
0.75
Mean
K
=
4
Cutoff (K) Area
0.992421
0.07
s=
5
0.8 0.137656
area
above
0,92
=
6 Malignant tumor
0.81
0.007579
0.158655
0.92
7 Mean K =
0.82
0.181651
0.11
s=
8
area below 0.845 =
0.83 0.206627
9
0.912632
0.84 0.233529
10
0.85
0.26227
area above 0.845 =
11
0.845 0247677
0.087368
12
13
H6 = NORMDIST(G6,!5BS7.SBS8.TRUE)
14 E5 = NOR WDIST(0.92,$B$4,$B$f >,TRUE)
15 E7 = 1 - E5
26
Chapter 4
(c) In column G, we vary the value of AT and compute the area above K under the
curve for people with malignant tumors in column H. We search for the
value of AT that gives an area of 0.25, which means that 25% of people with
tumors will not be identified. The value 0.84 gives an area of 0.233 5 and the
value 0.85 gives an area of 0.262 3. By trial and error, we find that K = 0.845
gives an area near 0.25.
In cell E10, wc insert K = 0.845 into the NORMDIST function for healthy
people and find that the area below K = 0.845 is 0.912 6. The area above K =
0.845 is I -0.912 6 = 0.087 4. That is, 8.7% of healthy people will produce a
false positive result, indicating the presence of a tumor.
4-6.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
27
?3
24
2b
26
27
28
29
30
31
32
A
B
C
Gaussian curve for light I julbs
mean845.2
sld dev =
94.2
total bulbs =
4768
bulbs per bar
20
sqrt(2pi)=
2.506628
D
E
F
G
x (hours) y {bulbs)
Formula for cell C2 ~
500
0.49 ($A$8*$A$ 10/($AS6*$A$12))*
1.25
525
EXP(-((B4- EA$4)"2)/(2*$y \$6A2))
550
2.98
13.64
600
625
26 28
450
47.19
650
675
78.95
400 '
rfX
700
123.11
725
178.92
Y V
350
750
242.35
F 9
305 94
775
<5 300
p
\
800
359 94
ë 250
/
\
825
OîW.OO
o
845.2
403.85
S 200
403.33
850
875
384.14
z 150
900
340.99
925
282.09
100
950
217.50
50
975
156.29
1000
104.67
0
1025
65 33
°°^
400
600
800
1000
1050
38.00
1075
20.60
Lifetime (h)
1100
10.41
1125
4.90
1150
2.15Í
1175
0.88
0.34
1200
f
i
1
Y
Y
\\
H
\
y
\
T«0(
1200
27
Statistics
4-7.
Use the same spreadsheet as in the previous problem, but vary the standard
deviation. Here arc the results:
Std. Dev.
_ 600
•fc 400
600
4-8.
800
1000
Hours
1200
A confidence interval is a region around the measured mean in which the true
mean is likely to lie: If we were to repeat a set of n measurements many times and
compute the mean and standard deviation for each set, the 95% confidence
interval would include the true population mean (whose value we do not know) in
95% of the sets of« measurements.
4-9.
Since the bars are drawn at a 50% confidence level, 50% of them ought to include
the mean value if many experiments are performed. 90% of the 90% confidence
bars must reach the mean value if we do enough experiments. The 90% bars must
be longer than the 50% bars because more of the 90% bars must reach the mean.
4-10.
Case 1: Comparing a measured result to a "known" value. See if the known value
is included within the 95% confidence interval computed as in Equation 4-7.
Case 2: Comparing replicate measurements. Use Equations 4-8 and 4-9 if the two
standard deviations are not significantly different from each other. Use
Equations 4-8a and 4-9a if the standard deviations arc significantly different.
Use the F test to decide if the two standard deviations arc significantly
different.
Case 3: Comparing individual differences. (Use Equations 4-10 and 4-11.)
4-11.
x = 0.14g, s = 0.03 4
90% confidence interval = 0.14 8 ±
99% confidence interval • 0.l4g±
(2.015)(0.034)
7=
= 0.14g±0.02 8
(4.032)(0.034)
= O.I4 8 ±0.05 6
28
4-12.
Chapter 4
99% confidence interval = x±^
r
= x ±000010
V7
(1.527 83 to 1.52803)
4-13.
(a) dL = deciliter = 0.1 L = 100 mL
(b) ^calculated - (0.053/0.042)2 = I.S9 < Ftóbie = 6.26 (for 5 degrees of freedom
in the numerator and 4 degrees of freedom in the denominator).
Since /-^calculated < Stable, we can use the following equations:
Spooled
t =
. y^ ; y . 0484
|14.5 7 -13.9 5 |
6-5
\ i g 5 = 2.12 < 2.262 (listed for 95% confidence and
9 degrees of freedom). The results agree and the trainee should be released.
4-14.
A
B
C
1 Comparison of two methods
2
3
Sample Method 1 Method 2
4
A
0.88
0.83
5
B
1.15
1.04
6
C
1.22
1.39
7
D
0.93
0.91
1.17
8
E
1.08
9
F
1.51
1.31
10
mean =
11
stdev =
12
tcalculoled
_
13
t:.::.- -
D
d.
0.05
0.11
-0.17
0.02
0.09
0.20
0.050
0.124
0.987
2.571
E
I
F
- B4-C4
= AVERAGE(D4:D9)
= STDEVf D4:D9)
= D10/D11*SQRT(6)
= TINV(0.05,5)
/«icntaed = 0.987 < 2.571 (Student's/for 95% confidence and 5 deg of freedom)
The difference is not significant.
4-15.
In the following spreadsheet, we find /calculated (which is labeled t Stat in cell F10)
is less than /tabie (t Critical two-tail in cell F14). Therefore, the difference
between the methods is not significant.
The probability P(T<=t) two-tail in cell F13 is 0.37. There is a 37% chance of
finding the observed difference between equivalent methods by random variations
in results. The probability would have to be <0.05 for us to conclude that the
methods differ.
29
Statistics
C
A
I
B
1 Paired t tost
2
Method 2
Sample
Method 1
3
0.83
0.86
A
4
1.04
1.15
B
5
1.39
1.22
6
C
0.91
0.93
7
D
1.08
1.17
8
E
1.31
1.51
F
g
10
11 Calculated t S tatistic in cell F10is
12 less than cnti<» I t In oel F14.
13 Thorfore, the différence between the
14 methods is nc if significant. |
4-16.
I D
E
F
t-Test: Paired Two Sample for Means
G
Variable 1
Variable 2
Mean
1,14333333 1.09333333
Variance
0.05118667 0.04818667
6
Observations
6
Pearson Correlation
0.84541418
Hypothesized Mean Difference
0
5
df
tStal
| 0.98692754
P{T<=t) one-tell
0.18449929
t Critical one-tail
2.01504918
P(T<=t) two-tail
0.36899857
t Crilical two-taH
|T!67BB??H
Fcalcula,ed = * 2 W = (0.039)2/(0.025)2 = 2.43
Stable = 9.28 for 3 degrees of freedom in the numerator and denominator
Since recalculated < Cable, the difference in standard deviation is not significant and
we use Equations 4-8 and 4-9.
¡sr\ni-\)
+ S22(n2-l)
¿pooled-M
,M+M2_2
mm
_ 1*1 -X2
'calculated
•pooled \jn\+n2
-^F
/0.025
4+ 4-2
11.382- 1.3461
0.032 8
0.032 8
/4«4
V4 + 4 = 1.55
/table (4 + 4 - 2 = 6 degrees of freedom) = 2.447
Since /calculated < /table, the difference is not significant.
4-17.
For Method 1, we compute x\ = 0.082 6052, ¿l = 000° °1 3 4.
For Method 2, x2 - 0.082 OO5, s2 - 0.000 129.
The two standard deviations differ by approximately a factor of 10. We should
use the F test to compare the two standard deviations:
Calculated " *22/*l2 = (0.000 12o)2/(0.000 013 4 ) 2 = 92.7
Cable = 6-26. Since Calculated > Cable, we use Equations 4-8a and 4-9a.
The following spreadsheet shows that /calculated =11.3 and /^ble =s 2.57.
/calculated > /tabic, so the difference is significant at the 95% confidence level.
30
Chapter 4
Paired t test
t-Test: Two-Sample Assuming unequal Va riances
Method 1 Method 2
0.082601
0.08183
0.08186
0.082621
0.08205
0.082589
0.082617 0.08206
0.082598
0.08215
0.08208 i
Mean
Variance
Observations
Hypothesized Mean Difference
df
tStat
P(T<=t) one-tail
t Critical one-tail
P(T<=t) two-tail
t Critical two-tail
Variable 1 Variable 2
0.082605 0.082005
1.8E-10 1.67E-08
5
6
0
5
11.31371 ^ — /calcula
11.3
4.72E-05
2.015049
9.43E-05Î
2.570578 <— /table =
- 2.57
4-18.
90% confidence interval = x ± ^ — p — ^ = x ± 1.1 R% < 1.2%.
v^
The answer is yes.
4-19.
For indicators 1 and 2: Fcaiculatcd = (0.002 25/0.000 98)2 = 5.27 > Ftabie ~ 2.2
(for 27 degrees of freedom in the numerator and 17 degrees of freedom in the
denominator). Since /'calculated > Cable, we use the following equations:
Degrees of freedom =
(s\2/n\
+S22ln2)2
(si2/*l)2
n\ - 1
Js2Vn2)2
H2-
(0.002 252/28 + 0.000 982/18)2
(0.002 252/28)2 (0.000 982/18)2 = 39.8 ~ 40
28-1
18-1
/calculated ~
F l -*2\
• \ t ó » l + sj/n2
10.095 65 - 0.086 86|
= = 18.2
V0.002 252/28+ 0.000 982/18
This is much greater than t for 40 degrees of freedom, which is 2.02.
The difference ]s significant.
For indicators 2 and 3: F ca i cu i alcd - (0.001 13/0.000 98)2 = 1,33 < Ftabie a 2.2 (for
28 degrees of freedom in the numerator and 17 degrees of freedom in the
denominator). Since Fca|cu|ated < F^bie, we use the following equations:
/ V ( n | - l ) + *2 2 (*2-l)
- 0.001075 8
n\ +«2-2
•Spooled
/calculated
_
*\-x2
•spooled
\! nl + "2 = 1.39 < 2.02
difference is not significant.
31
Statistics
4-20.
^.0 2 (31) + 29.82(31)
Spooled = M
32 + 3 2 - 2
-Ap
=
„_
299
52.9-31.4
39 + 32 = 2.88. The table gives / for 60 degrees of freedom,
' ~
29.9
which is close to 62. The difference is significant at the 95 and 99% levels.
4-21.
x = 97.0o,
J = 1-66
ts
95% confidence interval = x±~ñ
= 97.0o±
(2.776)(1.66)
~fe
" 97.0 0 ±2.0 6
Range = 94.94 to 99.0 6
The 95% confidence interval does not include the certified value of 94.6 ppm, so
the difference ]s significant at the 95% confidence level.
If we make one more measurement, the results are x = 96.5g, s = 1.8o
(2.57 IXI.80)
95% confidence interval = 96.5g±
7=
= 96.5s ± I.89
Range = 94.6g to 98.47
The 95% confidence interval still does not include the certified value of 94.6 ppm,
so the difference is still significant at the 95% confidence level.
4-22.
(a) Rainwater:
Calculated - (O.008/0.OO5)2 = 2.56 < Ftabie = 4.53 (for 4 degrees of freedom in
the numerator and 6 degrees of freedom in the denominator). Since
Calculated < Cable, we use the following equations:
- ^ 0 0 0 5 2 ( 6 ) + 0.0082(4)
•Spooled
/calculated -
=
Q
^
0.069-0.063 . ¡TI
.
, .
_ 0„ñ
0.OO637 A/7 + 5 ~ '-61 /lablc " 2"¿Á*
The difference is not significant.
Drinking water:
Fcalculated = (0.008/0.007)2 = 1,31 <
FtMe
= 6.39 (for 4 degrees of
freedom in the numerator and 4 degrees of freedom in the denominator).
Since emulated < Cable, we use the following equations:
_ ^0.007^4^0.0082(4) .
Q
^
¿pooled
t =
0.08
0.00752
\ITT7
• 1.89 < 2.306. The difference is not significant.
32
Chapter 4
(b) Gas chromatography:
/0.0052(6) + 0.0072(4)
¿pooled = \j
7+5^2—
= 0.00588
Ä
'
=
0.078 - 0.069 /7^7
>88
\/7 + 5
0.00588
=
2.61 > 2.228. The difference ]s significant.
Spectrophotometry
.
/ÔT0082(4) + 0.0082(4) =
•Spooled = " \ / ~
'
4-23.
5+
5
_' 2
_ 0.087 - 0.063
/5-5
O.OO800
V5 + 5
=
O.OO800
4-74 > 2.306. The difference ]s significant.
x - 201.8; S - 9.34
(/calculated = |216-201.81 / 9.34= 1.52
Gtablc = 1 672 for five measurements
Because C7Calcuiated < Stable, we should retain 216.
4-24.
Slope =-1.298 72 x 104 (±0.001 3190 x 104)
= -1.299 (±0.001) x lO4
or
_].298 7 (±0.0013)x lO4
Intercept - 256.695 (±323.57) = 3 (±3) x 102
4-25.
xi
0
2
3
sums: 5
m
'
y\
^iVi
X?
0
4
9
13
0
4
9
13
1
2
3
6
di
0.07143
-0.21429
0.142 86
0
n lUiVi) - ¿Zx\ ¿Zy\ 3x 1 3 - 5*<S
9
2
' 14 - 0.642 86
3x 13 - 5 2
rtZ{x?)~ (2^i)
% 2 ) ^ i - - ICviy¡) Ix;
n Z.(xi2) (Zx¡)2
13 = 6 - 13^5
3 * 13 - 5 2 "= T4 = 0.92857
A
0.005 10
0.045 92
0.02041
0.07143
33
Statistics
= 0.267 26
= sy^ß
3
= (0.267 26r\J^ = 0.12371
A Ilia
A3
*b = -sy \ / - 7 J - = (0.267 2 6 ) ^ 7 4
= 0.257 54
o
slope = O.64 ±0.12
4-26.
intercept = 0.93±0.2é
6
y =0.6154x + 1.3462
4-27.
3.0
10.0
20.0
30.0
40.0
-0.074
-1.411
-2.584
-3.750
-5.407
LINEST output
m -0.13789 0.195343 b
s,., 0.006635 0.162763 sb
11
1?
0.993102
0.197625
\2
cells B9:C 11
ilHighlight
Type
15 "=LINEST(B2:B6.A2:A6,TRUE,TRUE)"
10 Press CTRL+SHIFT+ENTER (on PC)
17 Press COMMAND+RETURN (on Mac)
-
34
Chapter 4
4-28.
We must measure how an analytical procedurerespondsto a known quantity of
analyte (or a known quantity of a related compound) before the procedure can be
used for an unknown. Therefore, we must be able to measure out the analyte (or a
related compound) in pure form to use as a calibration standard.
4-29.
Hopefully, the negative value is within experimental error of 0. If so, no
detectable analyte is present. If the negative concentration is beyond experimental
error, there is something wrong with your analysis. The same is true for a value
above 100% of the theoretical maximum concentration of an analyte. Another
possible way to get values below 0 or above 100% is if you extrapolated the
calibration curve past the range covered by standards, and the curve is not linear.
4-30.
Corrected absorbancc = 0.264 - 0.095 = 0.169
equation of line: 0.169 = 0.01630 x + 0.004 7 => x= 10.1 pg
A„
,.
y-b
4-31. (a) x - V
2.58-1.35
=
o,6l5
= 2.00
J> = (2 + 3 + 4 + 5)/4 = 3.5
* = (1 + 3 + 4 + 6)/4 = 3.5
2(x¡--)2 s (|_3.5)2 + ( 3 _ 3 5 ) 2 + ( 4_3 45) 2 + ( 6 _3 i 5 ) 2 =
T +
k
,
+
»
_QLZÜ2
m2Z(xi~x)2
0.196 12 . h \ _
(2.58-3.5)2
- 10.615 381 V I + 4 +(0.615 38)2 (13.0) - 0.38
Answer: 2.0o±0.38
(b) For k = 4 replicate measurements,
0.196 12
h I
• 10.615 381 \ / 4 " f 4
Answer: 2.0o±0.26
+
(2.58-3.5)2
(0.615 38)2 (13.0) - 0.26
]30
35
Statistics
4-32.
Mean absorbance = (0.265 + 0.269 + 0.272 + 0.258)/4 = 0.2660
Mean blank: (0.099 + 0.091 + 0.101 + 0.097)/4 - 0.0970
=> Corrected absorbance = 0.266o - 0.097o - 0.1690
Cells B30 and B3 lof the spreadsheet show that there are 10.1 ± 0.2 ug protein.
r^~r
I
Least-Squares Spreadsheet
0.35
10
11
12
13
14
15
16
17
18
19
20
21
10
10
10
15
15
20
20
20
-0.0003
-0.0003
0.0007
0 0857
0.0877
0.0887
0.1827
0.1727
0.1727
0.2457
0.2477
0 3257
0.3257
0.3307
LINEST output:
m 0.01630 0.00470
Sm 0.00022 0.00263
0.99785 0.00588 s.
22
23
24
25 Mean y =
26 £(x¡ - mean f
27
28 Measured y =
Number of
replicate
measurements
29 ofy(k) =
30 Derived x
31 Sx =
14 B24 = COUNT(B4:B17)
0.16184 B25 = AVERAGE(C4:C17)
723.214 B26 = DEVSQ(B4:B17)
0.169 Input
y = 0.0l63x + 0.0047
P
0.3
0.25
U.2
of
015
/
0.1
I
0.05
/
10
15
Protein (ug)
Input
10.082 B21 = (B28-C20VB20
0.2045' B31 = ((C22/B20),SQRT((1 /B29)*( 1 /B24)^((B28-B25)A2 V(B20*2-B26))
2Ü
36
Chapter 4
4-33. (a)
A
B
C
1 Least-Squares Spreadsheet
2
3
X
YcoTBOIed
4
5
6
0
0.062
0.122
7
8
9
10
11
1?
13
14
Srr,
15
R'
16
17
18
19
20
21
0.486
0.971
1.921
m
n =
Mean y =
E(x,- mean x) y =
Measured y =
Number of
replicato
measurements
22 ofy(k) =
2¿ Derived x
24 s « =
D
F
y
0
38.4
86.5
184.7
378.4
803.4
1662.8
9.1
47.5
95.6
193.8
387.5
812.5
1671.9
LINEST c
869.13 -22.0852 b
10.6422
¡1.9474 St,
0.9993
E
18.0527 sy
|
1600
G
|
H
1
y = 869.13x- 22.085
P
1400
1
i?nn
•g 1000
¡2 800
J?
w
600
400
200
7 B17 = COUNT(B4:B10)
450.6 B18 = AVERAGE<C4:C10)
2.87757 B 1 9 - D E VSQ(B4;B10)
1
0}
I
V&
u
¡J.t
145.0 Input
U.O
l.C
I.O
Methane (vol%)
—^-^^^
4 Input
0.19224 B23 = (B21-C13)/B13
0.0137 B24 = (C15/B13)*SQRT((1/B22K1/B17)+((B21-B18) A 2y(B13"2*B19))
(b) Corrected signal = 154.0 -9.0 = 145.0 mV
(c) Cells B23 and B24 give [CH4] = 0.192 (±0.014) vol%
4-34.
0.350 - -1.17 x 10- 4 * 2 + 0.018 58*-0.000 7
1.17 x 10-4 x2 - 0.018 58 x + 0.350 7 -- 0
2
4
= +0.01858 W 0 . 0 1 8 5 8 - 4 ( 1 . 1 7 * 10 ) (0.3507)
- 137 or 21.9 pg
*"
2(1.17x10-4)
Correct answer is 21.9 pg.
I
¿
37
Statistics
4-35.
(a) The logarithmic graph spreads out the data and is linear over the entire range.
i
r
Linear plot
<
s
Log plot
4000
F.
3000
O
2000
y = 17.063X + 32.183
_l
100
I
200
300
0.969X +1.339
400
-1
p-Nitrophenol (pg/mL)
0
1
log (pg/mL)
(b) log (current, nA) = 0.969 2 log (concentration, pg/mL) + 1.339
(c) log (99.9) = 0.969 2 log [X] + 1.339
=> log [X] = 0.681 6 => [X] = 4.80pg/mL
4-36.
For 8 degrees of freedom, tgo% = 1.860 and ¿99% = 3.355.
90% confidence interval: 15.22 (±1.860 x 0.46) = 15.22 ± 0.86 pg
99% confidence interval: 15.22 (±3.355 x 0.46) = 15.2 ± I.5 P-g
CHAPTER 5
QUALITY ASSURANCE AND CALIBRATION METHODS
5-1.
Get the right data: Measure what is relevant to the question at hand.
Get the data right: Sampling and analytical procedures must be satisfactory to
measure what we intend to measure.
Keep the data right: Record keeping should document that samples were
collected properly and data has demonstrated reliability.
5-2.
The three parts of quality assurance are defining use objectives, setting
specifications, and assessing results.
Use objectives:
Question: Why do I want the data and results and how will I use them?
Actions: Write use objectives.
Specifications:
Question: How good do the numbers have to be?
Actions: Write specifications and pick an analytical method to meet the
specifications. Consider requirements for sampling, precision, accuracy,
selectivity, sensitivity, detection limit, robustness, and allowed rate of false
results. Plan to employ blanks, fortification, calibration checks, quality control
samples, and control charts. Write and follow standard operating procedures.
Assessment:
Question: Did I meet the specifications?
Actions: Compare data and results with specifications. Document procedures
and keep records suitable for meeting use objectives. Verify that the use
objectives were met.
5-3.
Precision is demonstrated by the repeatability of analyses of replicate samples
and replicate portions of the same sample. Accuracy is demonstrated by
analyzing certified reference materials, by comparing results from different
analytical methods, by fortification (spike) recovery, by standard additions, by
calibration checks, blanks, and quality control samples (blind samples).
5-4.
Raw data are directly measured quantities, such as peak area in a chromatogram
or volume from a buret. Treated data are concentrations or amounts found by
applying a calibration method to the raw data. Results, such as the mean and
standard deviation, are what we ultimately report after applying statistics to
treated data.
38
Quality Assurance and Calibration Methods
39
5-5.
A calibration check is an analysis of a solution formulated by the analyst to
contain a known concentration of analyte. It is the analyst's own check that
procedures and instruments arc functioning correctly. A performance test sample
is an analysis of a solution formulated by someone other than the analyst to
contain a known concentration of analyte. It is a test to see if the analyst gets the
right answer when he or she does not know the right answer.
5-6.
A blank is a sample intended to contain no analyte. Positive response to the
blank arises from analyte impurities in reagents and equipment and from
interference by other species. A method blank is taken through all steps in a
chemical analysis. A reagent blank is the same as a method blank, but it has not
been subjected to all sample preparation procedures. A field blank is similar to a
method blank, but it has been taken into the field and exposed to the same
environment as samples collected in the field and transported to the lab.
5-7.
Linear range is the analyte concentration interval over which the analytical signal
is proportional to analyte concentration. Dynamic range is the concentration
range over which there is a useable response to analyte, even if it is not linear.
Range is the analyte concentration interval over which an analytical method has
specified linearity, accuracy, and precision.
5-8.
A false positive is a conclusion that analyte exceeds a certain limit when, in fact,
it is below the limit. A false negative is a conclusion that analyte is below a
certain limit when, in fact, it is above the limit.
5-9.
- 1 % of the area under the curve for blanks lies to the right of the detection limit.
Therefore, - 1 % of samples containing no analyte will give a signal above the
detection limit. 50% of the area under the curve for samples containing analyte at
the detection limit lies below the detection limit. Therefore, 50% of samples
containing analyte at the detection limit will be reported as not containing analyte
at a level above the detection limit.
5-10.
A control chart tracks the performance of a process to sec if it remains within
expected bounds. Six indications that a process might be out of control are (1) a
reading outside the action lines, (2) 2 out of 3 consecutive readings between the
warning and action lines, (3) 7 consecutive measurements all above or all below
the center line, (4) six consecutive measurements, all steadily increasing or all
Chapter 5
steadily decreasing, wherever they arc located, (5) 14 consecutive points
alternating up and down, regardless of where they arc located, and (6) an obvious
nonrandom pattern.
5-11.
Statement (c) is correct. The purpose of the analysis is to see if concentrations are
in compliance with (in other words, do not exceed) levels set by a certain rule.
5-12.
The instrument detection limit is obtained by replicate measurements of aliquots
from one sample. The method detection limit is obtained by preparing and
analyzing many independent samples. There is more variability in the latter
procedure, so the method detection limit should be higher than the instrument
detection limit.
Robustness is the ability of an analytical method to be unaffected by small,
deliberate changes in operating parameters. Intermediate precision is the
variation observed when an assay is performed by different people on different
instruments on different days in the same lab. Each analysis might incorporate
independently prepared reagents and different lots of the same chromatography
column from one manufacturer. When demonstrating intermediate precision,
experimental conditions are intended to be the same in each analysis. When
measuring robustness, conditions are intentionally varied by small amounts.
5-13.
Instrument precision, also called injection precision, is the reproducibility
observed when the same quantity of one sample is repeatedly introduced into an
instrument.
Intra-assay precision is evaluated by analyzing aliquots of a homogeneous
material several times by one person on one day with the same equipment.
Intermediate precision is the variation observed when an assay is performed by
different people on different instruments on different days in the same lab.
Interlaboratory precision is the reproducibility observed when aliquots of the
same sample arc analyzed by different people in different laboratories at different
times using equipment and reagents belonging to each lab.
5-14.
Criteria:
• Observations outside action lines — no
• 2 out of 3 consecutive measurements between warning and action lines — no
41
Quality Assurance and Calibration Methods
• 7 consecutive measurements all above or all below the center line — YES:
observations 2-10 (starting from the left side) are all below the center line
• 6 consecutive measurements steadily increasing or steadily decreasing — no
• 14 consecutive points alternating up and down — no
• Obvious nonrandom pattern — no
5-15.
LINEST gives m, b, sm, s^ Ä2, and sy in cells B19:C21. TRENDLINE produces the
same value of m, b, and R2, which are printed inside the graph. The 95%
confidence interval for>> is computed in cell C24.
A
B
|
C
D
1 Graphing data with random Gaussian noise
2 Generating equation: t - ¿ 0 . 4 X + 1 of
80
3 Gaussian noise =
4
X
5
y
14
6
0
350
10
7
566
20
8
30
957
9
1067
40
10
1354
11
50
60
1573
12
1732
13
70
80
2190
14
2330
90
15
100
2508
16
17
18
LINEST output
m 24.80727 89.72727 b
19
20
Sm 0.683532 40.43827 s,.
21
1?
0.993214
22
23
Studenrs t =
24
t*s>
5-16.
I
I
H
G
F
E
~~|
2500
^^^^_
y = 24.807X t 89.727
R* = 0.9932
2000
1500
1000
500
Y^u y
0
Error bars are ± t*s,, in cell C24
20
40
60
80
100
X
71.6894 s y
2.262159 =TINV(0.0 5.9)
16:-' 1728 =C23 'C21
(a) For the fortification level of 22.2 ng/mL, the mean of the 5 values is 23.6f,
ng/mL and the standard deviation is 5.63 ng/mL.
5.63
Precision = 100 x 23.66
Accuracy - 100 x
8%.
23.66 - 22.2
= 6.6%
For the fortification level of 88.2 ng/mL, the mean of the 5 values is 82.4g
ng/mL and the standard deviation is 11.4o. ng/mL.
11.49
Precision = 100 x 82.48 = 13.9%.
42
Chapter 5
Accuracy = 100 x
j ^
= -6.5%
For the fortification level of 314 ng/mL, the mean of the 5 values is 302.8
ng/mL and the standard deviation is 23.5] ng/mL.
23.51
Precision = lOOx ^ " g = 7.8%.
Accuracy = 100 x
302 8 — 314
jj^
= -3.6%
(b) Standard deviation of 10 samples: J = 28.2 ; mean blank: >^ a n k = 45.o
Signal detection limit =>^]ank + 35 - 45.o + (3)(28.2) = 129.6
Concentration detection limit = ~ = TZi—mow i =4.8 * 10"8 M
m
1,75 x 10y M"1
,. . *
. .
10s
(10X28.2)
Lower limit of quantitation - — - t 7 5 x | Q 9 M - I = J-6 * 10 ~ 7 M
5-17.
(a) 1 wt% => C=0.01: CV(%) * 2(I-°-5iog0.0i) _ 2 2 = 4%
If C = 10-12, CV(%)
Ä 27
= 128%.
(b) If class CV is 50% of the value given by the Horwitz curve, it would be 0.5 x
2(1-0.5 logo. 1) = i.4o/o
5-18.
Mean - OJ83 pg/L and standard deviation = 0.0214 pg/L
0.383 Pg/L
% recovery = 0 4 0 u g / L x 100 - 96%
The measurements are already expressed in concentration units. The
concentration detection limit is 3 times the standard deviation • 3(0.0214 pg/L) =
0.064 pg/L.
5-19.
The low concentration of Ni-EDTA has a standard deviation of 28.2 counts for
10 measurements. The detection limit is estimated to be
7dl =yblank + 3í = 45 + 3(28.2) = 129.6 counts
To convert counts to molarity, we note that a 1.00 pM solution gave a net signal
of 1797 - 45 = 1752 counts. The slope of the calibration curve is therefore
estimated to be
m
^sample -.Vblank
_ 1797-45
" sample concentration = 1.00 pM
The minimum detectable concentration is
! 175
. counts
2 x 10- " M
43
Quality Assurance and Calibration Methods
35
m
5-20.
_i3JÍ2A21cpunts_
1.752* lO^counts/M
=
8
For a concentration of 0.2 pg/L, the relative standard deviation of 14.4%»
corresponds to (0.144)(0.2 pg/L) = 0.028 8 pg/L. The detection limit is 3(0.028
8 pg/L) = 0.086 pg/L. Here are the results for the other concentrations:
Concentration Relative standard Concentration standard
deviation (pg/L)
deviation (%)
(pg/L)
5-21.
limit (pg/L)
0.086
0.102
0.096
0.028 8
0.034 0
14.4
6.8
3.2
1.9
0.2
0.5
1.0
2.0
Detection
0.032 0
0.114
0.038 0
mean detection limit 0.10
If an athlete tests positive for drugs, the test should be repeated with a second
sample that was drawn at the same time as the first sample and preserved in an
appropriate manner. If there is a 1% chance of a false positive in each test, the
chances of observing a false positive twice in a row arc 1% of 1% or 0.01%.
Instead of falsely accusing 1% of innocent athletes, we would be falsely accusing
0.01% of innocent athletes.
5-22.
Comparison of Lab C with Lab A:
First, use the F test to see if the standard deviations are significantly different:
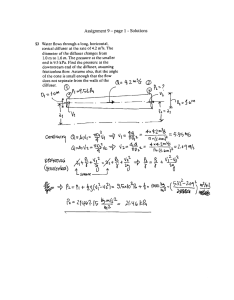
Fcaiculatcd = ^ A " 0.782/0.142 = 31 .n > F ub le - 3.88 (with 2 degrees of
freedom for.se and 12 degrees of freedom for s\)
Standard deviations are not equivalent, so use the following t test:
(siyn\+s2Vn2)2
(0.142/13 + 0.782/3)2
Degrees of freedom - {s{ym)2
(s2Vn2)2 '" (0.142/13)2 (0.78^/3)2
-^rr + ~ ^ r
'CalCUlated
1*1-*2l
" ^s2/nl+4/n2
13 1
+
ll.59-2.68l
" V0.142/13 + 0.782/3
¿U3
'
3-1
' '
For 2 degrees of freedom, ruble • 4.303 for 95% confidence. Since /calculated <
'table. w e conclude that the difference between Lab C and Lab A is not
significant.
z
44
Chapter 5
Comparison of Lab C with Lab B:
^calculated = ¿c^B = 0.782/0.562 = 1.94 < F^bie = 4.74 (with 2 degrees of
freedom for sc and 7 degrees of freedom fori A)- The standard deviations are not
significantly different, so we use the following t test:
¿pooled - \l
'calculated
—
/0.562 (8 - 1 ) + 0.782(3-1)
...
8+ 3-2
= 0.616
11.65-2.681
0 61 A
'table • 2.262 for 95% confidence and 8 + 3 - 2 = 9 degrees of freedom,
'calculated > 'table, so the difference is significant at the 95% confidence level.
It makes no sense to conclude that Lab C [2.68 ± 0.78 (3)] > Lab B [1.65 ± 0.56
(8)], but Lab C = Lab A [1.59 ± 0.14 (13)]. The problem with the comparison of
Labs C and A is that the standard deviation of C is much greater than the standard
deviation of A and the number of replicates for C is much less than the number of
replicates for A. The result is that we used a large composite standard deviation
and a small composite number of degrees of freedom. The conclusion is biased
by a large standard deviation and a small number of degrees of freedom. I would
tentatively conclude that results from Lab C are greater than results from Labs B
and A. I would also ask for more replicate results from Lab C. With just 3
replications, it is hard to reach any statistically significant conclusions.
5-23.
A small volume of standard will not change the sample matrix very much, so
matrix effects remain nearly constant. If large, variable volumes of standard are
used, the matrix is different in every mixture and the matrix effects will be
different in every sample.
5-24.
(a) [Cu2+]f = [Cu2+]j-pj; = 0.950 [Cu2+]j
(b) [SJf = [S]if[ = (100.0ppm) doao'mLJ =
{C)
[Cu2+jj
1.00 ppm+ 0.950[Ct|2+]¡
==
0.262
„ ,+i
0.500 => t C u *
100
=
PP m
1-04 ppm
45
Quality Assurance and Calibration Methods
5-25.
(a) All solutions were made up to the same final volume. Therefore, we prepare
a graph of signal versus concentration of added standard. The line in the
graph was drawn by the method of least squares with the following spreadsheet. The jc-intercept, 8.72 ppb, is the concentration of unknown in the 10mL solution. In cell B27 of the spreadsheet (on the next page), wefindthe
standard deviation of the jr-intercept to be 0.427 ppm. Areasonableanswer
is 8.72 ± 0.43 ppb.
y = 3.l36x + 27.36
2
en
Intercept =
-8.72 ng/mL
(7)
-10
Added Sr (ng/mL)
(b) Unknown solution volume • 10.0 mL with Sr = 8.72 ppb = 8.72 ng/mL. In
10.0 mL, there are (10 mL)(8.72 ng/mL) = 87.2 ng. Solution was made from
0.750 mg of tooth enamel. Sr (ppm) in tooth enamel is
mass of Sr
0
Concentration (ppm)
mass of enamel
87.2xlQyg
6
— -J= - x l 0
0.750xlO' g
116 ppm
(c) Relative uncertainty of intercept is 100 x 0.43/8.72 = 4.9%, which leads to a
4.9%» uncertainty in the concentration of Sr in the tooth enamel. 0.049 x 116
ppm = 5.7 ppm. Final answer: 116 ±6 ppm.
(d) Student's /for « - 2 = 5 - 2 = 3 degrees of freedom and 95% confidence is
3.182. We found standard deviation = 5.7 ppm. 95% confidence interval is
+ ts - (3.182)(5.7 ppm) = 18.1 ppm. Answer: 116 ± 18 ppm.
Chapter 5
Spreadsheet for 5-25 (a). To execute LINEST, highlight cells B16-C18, enter
"=LINEST(B7:BI I.A7.A11,TRUE/TRUE", and press CONTROL + SHIFT
+ ENTER on a PC or COMMAND(3€) + RETURN on a Mac.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
A
B
C
D
I
E
Standard Addition Constant Volume Least-Squares Spreadsheet
X
y
Added Sr
(ng/mL)
0.00
2.50
5.00
7.50
10.00
Signal
28.000
34.300
42.800
51.500
58.600
B16:C18 = LINEST(B7:B11 ,A7:A11 .TRUE.TRUE)
LINEST output:
3.1360 27.3600 b
Sm
0.0945
0.5790 sb
R¿
0.9973
0.7474 sy
m
x-intercept = -b/m =
-8.7241
n=
Mean y =
Z(x¡ - mean x)2 =
5 B22 = COUNT(A7:A11)
43.040 B23 = AVERAGE(B7:B11)
62.5 B24 = DEVSQ(A7:A11 )
Std deviation of
x-intercept =
0.427
B27=(C18/ABS(B161 )*SQRT((1< B22)+ B23A2/(B16A2*B24))
(a) The intercept for tap water is -6.0 mL, corresponding to an addition of
(6.0 mL)(0.152 ng/mL) = 0.91 2 ng Eu(IIl). This much Eu(IH) is in 10.00
mL of tap water, so the concentration is 0.912 ng/10.00 mL = 0.091 ng/mL.
For pond water, the intercept of-14.6 mL corresponds to an addition of
(14.6 mL)(15.2 ng/mL) - 2.22 x ](fi n g /l0.00 mL pond water = 22.2 ng/mL.
(b) Added standard Eu(lll) gives a response of 3.03 units/ng for tap water and
0.0822 units/ng for pond water. The relative response is 3.03/0.0822 = 36.9
times greater in tap water than in pond water. There is probably a matrix
effect in which something in pond water decreases the Eu(III) emission. By
using standard addition, we measure the response in the actual sample
matrix. Even though Eu(III) in pond water and tap water do not give equal
47
Quality Assurance and Calibration Methods
signals, we measure the actual signal in each matrix and can therefore carry
out accurate analyses.
5-27.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
A
B
|
C
D
Standard Addition Constant Volume Least-Squares Spreadsheet
V total _
50
[S]|(M) =
2.64
Vo =
25.00
V,(mL) =
NaCI
standard
X
y
Concentration of
added NaCI
l(s+x) =
[Si
signal
added
0.000
1.000
2.000
3.000
4.000
3.13
5.40
7.89
10.30
12.48
0
0.0528
0.1056
0.1584
0.2112
C7 = $A$6*B7/$A$4
B16:D18 = LINEST(E7:E11.C7:C1 1,TRUE,TRUE)
LINEST output:
44.6970
0.5511
3m
tf
0.9995
3.1200 b
0.0713 Sb
m
x-intercept = -b/m =
n=
Mean y =
Z(X| - mean x)2 =
0.0920
Sy
-0 06980
5 B22 = COUNT(B7:B11)
7.84 B23 = AVERAGE(E7:E11)
0.0278784 B24 = DEVSQ(C7:C11)
Std deviation of
0.00235I
x-intercept =
A
B27=(C18/ABS(Bie >))*SQRT((1/EI22) + B23 2/(B16 *2*B24))
y = 44.697X
II
Intercept =
-0.069 8
/ .
-0.10
-0.05
0.00
0.05
0.10
x = [S],
0.15
0:20
0.25
48
Chapter 5
(a) All solutions arc made to constant volume, so we plot Is+x vs. [S]r. The
negative intercept is [X]r = 0.069 8 M. The initial concentration of NaCI is
larger by the dilution factor of 2 (50.00 mL/25.00 mL). The initial
concentration of NaCI in serum was 2.000 x 0.069 8 M = 0.013 96 M.
(b) The x-intercept is computed in cell B20 and its uncertainty in cell B27. The
relative uncertainty is (0.002 35)/(0.069 8) = 3.37%. This uncertainty is
much larger than the relative uncertainties in volume measurement, so the
uncertainty in the original concentration of Na + should be 3.37%.
A reasonable expression of [Na f ] in the original serum is 0.140 (±3.37%) M
= 0.140(±0.004 7 )M.
95% confidence interval = ± ts = ± (3.182)(0.0047 M) = ± 0.015 M, where t
is taken for 5 - 2 = 3 degrees of freedom.
A
B
C
D
1 Standard Addition Constant Volume Least-Squares Spreadsheet
2
3
X
y
4
Spike (mg/gj
l(s+x) =
5
[S],
signal
6
0.00
15.6
7
3.12
21.1
8
7.18
25.5
y
8.48
30.0
10
20.0
48.8
n
38.2
83.4
12
13 B16:D18 = LINEST(C6:C11,B6:B11,TRUE,TRUE)
14
15
LINEST output:
16
m
1.7776
14 5928 b
17
sm
0.0449
0.8190 «b
18
R*
0.9974
1.4246 Sy
19
20 x-intercept = -b/m =
-B.209O6
21
22 n =
6 B22 = COUNT(B<i:B11)
23 Mean y =
37.40 B23 = AVERAGE (C6:C11)
2
24 I(Xj - mean x) =
1004.7838 B24 = DEVSQ(B6:B11 )
25
26 Std deviation of
27 x-intercept =
0.62445
28 B27 =(C18/ABS(B16)j*SQRT(f1/B 22) + B23A2/(B16'*2*B24))
49
Quality Assurance and Calibration Methods
y=1.778x + 14.593
n
Intercept =
-8,21
-10
10
20
30
40
x = mg alliin/g of garlic
(a) In cells B20 and B27 of the spreadsheet, the negative x-intercept of the
standard addition graph is 8.21 ± 0.62 mg alliin/g garlic.
(b) Two moles of alliin (FM 177.2) produce one mole of allicin (FM 162.3) in
the assay. Therefore, the quantity of allicin in garlic is Vi{\ 62.3/177.2)(8.21
± 0.62 mg/g) - 3.76 ± 0.28 mg allicin/g garlic or 3.8 ± 0.3 mg allicin/g
garlic.
5-29.
Standard addition is appropriate when the sample matrix is unknown or complex
and hard to duplicate, and unknown matrix effects are anticipated. An internal
standard can be added to an unknown at the start of a procedure in which
uncontrolled losses of sample will occur. The relative amounts of unknown and
standard remain constant. The internal standard is excellent if instrument
conditions vary from run to run. Variations affect the analyte and standard
equally, so the relative signal remains constant. In chromatography the amount
of sample injected into the instrument is very small and not very reproducible.
However, the relative quantities of standard and analyte remain constant
regardless of the sample size.
5-30.
(a)
Ax
[X]
"®
(b) [S]
(c)
Ax
[X]
-(i
3 473
10222
= F [1.72 mM]
[3.47 mM]
1.00 mL
847 mM
UO.Oml
F=0.168 4
4431
5 428
•j) => [X] = 6 . 1 6 m M
0.168
4(j7X
847 mM
[X]
50
Chapter 5
(d) The original concentration of [X] was twice as great as the diluted
concentration, so [X] = 12.3 mM.
5-31.
For the standard mixture:
Í2L Jé£\
10-UA
J 15.3 M ^
_ A.M
== F
[X] " f{[S}) =* [0.800 mM]
L[0.500 mM]J => ^=0.4126
Chloroform added to unknown = (10.2 x 10-6 L)(l 484 g/L) = 0.015 I4 g =
0.126s mmol in 0.100 L = 1.26s mM
For the unknown mixture:
¿X
r,(AS\
8.7 // A
( 29.4 M Ï
F
=
4126
°- l[1.268mM]J => [X] = 0.909mM
M - lisiJ^ lX]
[DDT] in unknown - (0.909 mM) ( J ' Q Q ^ J = 9.09 mM
5-32.
Data in the following table arc plotted in the accompanying graph. If the
equation
area of analyte signal
area of standard signal
/'concentration of analyte >
^concentration of standard^
is obeyed, the graph should be a straight line going through the origin, which it
is. The slope, 1.0757, is theresponsefactor. Over the concentration ratio
analyte/standard = 0.10 to 1.00, the standard deviation of theresponsefactor in
the table is 0.0668 = 6.2%.
Sample
1
2
3
Concentration ratio
Area ratio
CioHg/CioDs
CioHg/CioDs
0.10
0.101
0.50
0.573
1.00
1.072
F=
area ratio/cone, ratio
1.0127
1-1461
1.0724
mean = 1.0757
standard deviation 0.06<s8
relative standard deviation 6.2%
51
Quality Assurance and Calibration Methods
1.0
0757X
0.8
o
| 0.6
«
i
<
0.4
0.2
0.0
0.0
0.2
0.4
0.6
08
1.0
Concentration ratio
5-33.
To use the internal standard, a known quantity of internal standard is added to a
sample of sewage. Analyte signal is measured relative to the signal from internal
standard. Since the internal standard is so similar to analyte (differing only by
the substitution of D for H), matrix effects are likely to be essentially identical for
both compounds. If matrix enhances the response to analyte, it enhances the
response to internal standard to the same extent. By measuring unknown relative
to the known injection of standard, an accurate measurement of unknown can be
made. The key is to use a standard that is almost identical to the analyte in
physical properties.
52
5-34.
Chapter 5
Molarities are given in row 12 of the following spreadsheet and uncertainties are
given in row 13:
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
A
B
C
Experimen tal Design
I
I
Volumes of unknown acids
A
B
C
2
2
0
2
2
0
2
2
2
2
D
(mL)
2
2
2
0
2
E
mmol
NaOH
3.015
1.385
2.180
2.548
3.140
[Cl
[B]
[A]
Molarity 0.25635 0.44035 0.83785
Std, dev. 0.026729 0.026729 0.026729
0.995942 0.063896
#N/A
R2
s>
Highlight aïllsC12:E14
Type "=Lir<IEST(D5:D9,A5:C9.FA -SEJRUE m
Press CTRL+SHIFT+E NTER(onl =C)
Press CONIMAND+RETURN (on f i/lac)
CHAPTER 6
CHEMICAL EQUILIBRIUM
6-1.
Concentrations in an equilibrium constant are really dimensionless ratios of actual
concentrations divided by standard state concentrations. Since standard states are
1 M for solutes, 1 bar for gases, and pure substances for solids and liquids, these
are the units we must use. A solvent is approximated as a pure liquid.
6-2.
All concentrations in equilibrium constants are expressed as dimensionless ratios
of actual concentrations divided by standard-state concentrations.
6-3.
Predictions based on free energy or Le Châtelier's principle tell us which way a
reaction will go (thermodynamics), but not how long it will take (kinetics). A
reaction could be over instantly or it could take forever,
6-4.
(a)
65
,
K - ^ -
6-6.
HOBr + OCT
^
HOCI + OBr"
Kl = 1/15
HOC1
^
H+ +
K7 = 3 . 0 * 1 0 - 8
^
H+ + OBr"
K=l/[Ag + ]3[P0 3 4 -]
HOBr
(b)
PC0\IPQX1'2
f3.6xlQ4Torr
barV
0
U0Torr/aUn'>- "atrnJ
(b) give off
OCT
6-7.
(a) Decrease
6_8<
K
6-9.
(a) Right because reactant is added
=
K =
K = KXK2 - 2.0 x 10-9
(c) negative
^59,0 * 103 J/mol)/(8.314472 J/(K-mol))(298.l5 K) = 5 x 10 1 '
(b) Right because product is removed
(c) Neither because solid graphite does not appear in the reaction quotient
(d) Right. The pressure of reactant and product both increase by a factor of 8.
However, reactant appears to the second power in the reaction quotient and
product only appears to the first power. Increasing each pressure by the same
factor decreases the reaction quotient.
(e) Smaller. An exothermic reaction liberates heat. Adding heat is like adding a
product.
53
54
Chapter 6
(a) ä - = / > H 2 0 = e-AG°/Ä7,= e-iAH° - TAS°yRT
6-10.
= e—{«63.11 x l03j/mol)-(298.15K)(148JK-t moH)]/(8.3l4 J K~' mol-l)(298.15 K)}
= 4.7 x lO^bar
(b) Pn20 - 1 • e-iW°-T*S°y/tT => AH° - TAS° must be zero.
AIP
A/T - TAS° = O => T = — = 426 K = 153°C
6-11.
(a) Let's designate the equilibrium constant at temperature T\ as K\ and the
equilibrium constant at temperature T2 as K2.
tf, = e-&G'/RT\ = Q-iAff - TIáS°)/RT\ = c-M/'/RTi,QÎiS°/R
Similarly, K2 = Q~^IRT2
• e^«
Dividing K\ by K2 gives j ¿ = e-(A//V/?)(l/7ï - 1/72)
1 Y1
n
Ki
Putting in K\ = 1.479 x 10-5 a t T] = 278.15 K and
K2 = 1.570 x 10-5 at 72 = 283.15 K gives Alf
= +7.82 kJ / mol.
(b) K = e-Ar//Är«eA5*//r
. „
A/yyn
\nK- --t\T)
y
w A:
AS+
T"
b
A graph of In A.' vs. MT will have a slope of -AIÍ/R
¿_„
ö-iz.
f
v
W
n
y
f 48.0 Pa f ,f 1370 Pa Y 3310 Pa ^
^i 0 5Pa/barJ / (jO* Pa/bar Al 05 Pa/bar J
= 5.08 x 10-4 < AT
The reaction will go to the right.
It was not really necessary to convert Pa to bar, since the units cancel.
H2
(b)
Initial pressure:
Final pressure:
+
Br2
Ç*
2HBr
1370
3 310
48.0
1370-A
3 310-JC
48.0 + 2x
Note that 2x Pa of HBr arc formed when x Pa of H2 are consumed.
(48.0 + 2x)2
(1370-x)(3310-^)
/>H2 = 1 366 Pa,
=
7
mm
-
2 x l < H
^
* = 4.50Pa
PBr2 - 3 306 Pa, PHBT • 57.0 Pa
55
Chemical Equilibrium
(c) Neither, since Q is unchanged.
(d) HBr will be formed, since AW is positive.
6-13.
The concentration of MTBE in solution is 100 pg/mL = 100 mg/L. The molarity
is [MTBE] = 88 15 J/mo\ = 1134 x 10 3 M. The pressure in the gas phase i s P
= [MTBEytfh = (1.134 x lO*3 M)/(1.71 M/bar) = 0.663 mbar.
6-14.
[Cu + ][Br] = Ksp
[Cu+][0.10] = 5 x 1 0 - 9 => [Cu+] = 5 x 10-8 M
6-15.
[Ag+]4[Fe(CN)t] = * s p
[1.0 x 10-6]4[Fe(CN)46-] = 8.5 x 10"45 => [Fe(CN)4¿] = 8.5 x 10-21 M = 8.5 zM
6-16.
If we let x = [Cu 2+ ], then [SO2*"] = y r
K = [Cu 2+ ] 4 [OH"]6 [SO4-] = (x)4 (1.0 x 10-6)6 ¿jc) = 2.3 x 10-69
6-17.
(a) [Zn2+]2[Fe(CN)t] = (0.000 10)2[Fe(CN)46] = 2.1 x 10'6
=> [Fc(CN)4¿] = 2.1 x 10-8 M
(b) [Zn 2+ ]2[Fe(CN)t] = (5.0 x 10- 7 ) 2 [Fe(CN)t] = 2.1 x I 0 1 6
=> [FeiCNjt] = 8.4 x 10-4 M
6-18.
BX2 coprecipitates with AX3. This means that some BX2 is trapped in the AX3
during precipitation of AX3.
6-19.
ForCaS0 4 , Ksp = 2A x 10-5.
For
Ag 2 S0 4 , Ksp = 15 * lO'5.
Removing 99% of the Ca 2+ reduces [Ca2+] to 0.000 500 M. The concentration of
S0 4 _ in equilibrium with O.OOO 500 M Ca 2+ is 2.4 x 10-5/0.000 500 = 0.048 M.
This much SO42- wiU precipitate Ag2S04, because Q - [Ag + ] 2 [SO4"] =
(0.0300) 2 (0.048) = 4.3 x 10"5 > Ksp. The separation is not feasible.
When Ag+ first precipitates, [S0 4 _ ] - 1 . 5 * 10"5/(0.0300)2 = 1.67 x 10-2 M.
[Ca 2+ ] = 2.4 x 10-5/1.67 x 10"2 = 0.001 4 M. 97% of the Ca 2+ has precipitated.
6-20.
BaCr0 4 (i) ^
Ba 2+ + Cr024"
Ksp = 2.1 x 10-10
Ag 2 Cr0 4 (s) ^
2Ag+ + C1O4
Ksp - 1.2 x 10-12
56
Chapter 6
The stoichiometrics are not identical, so it is not clear that the salt with lower Ksp
will precipitate first. Let's try each possibility. Suppose that BaCr04 precipitates
first. The concentration of C1O4" that will reduce Ba 2+ to 0.1% of its initial
concentration is
[Ba2+][Cr04"] = [LO x 10-5][CrO4] - 2.1 x 10-10 ^
[C1O4] = 2.1 x io-5
M.
Will this much Chromate precipitate 0.010 M Ag+? We test by evaluating the
reaction quotient for Ag2Cr04:
Ô = [Ag+]2[Cr04"] = (0.010)2(2.1 x 10-5) - 2.1 x lfr* > Ksp for Ag 2 Cr0 4
Since Q > Ksp for Ag2Cr04, Ag+ will precipitate.
Let's try the reverse calculation. If Ag2Cr04 precipitates first, the concentration
of C1O4" that will reduce Ag+ to 1.0 * 10"5M is
[Ag+]2[Cr024"] = [1.0 x 10-5]2[CrO24"] = 1.2 x 10-12 ^
[C1O4] = 0.012 M.
This concentration of C1O4 exceeds the concentration required to precipitate
99.90% of Ba2+. Neither Ag + nor Ba 2+ can be 99.90% precipitated without
precipitating the other ion.
6-21.
Salt
[Ag+]
Ksp
(M, in equilibrium with 0.1 M anion)
1.8 x 10-10
AgCl
AgBr
Agi
Ag 2 Cr0 4
5.0 x 10-13
8.3 x lO-' 7
1.2 x 10-12
ATsp/0.10
A:sp/0.10
Ksp/Q.\Q
•\]Ksp/0.\0
= 1.8x10-9
= 5,0x10-12
= 8.3x10-16
=
3,5 x 10-6
L requires the lowest concentration of Ag+ to begin precipitating, so F precipitates
first. The order of precipitation is:
6-22.
F before Br" before CI" before CrO2".
At low F concentration, [Pb2+] decreases with increasing [F] because of the
reaction Pb2"1" + 2I2" —» Pbl2(A). Concentrations of other Pb 2 + - F species arc
negligible. At high F concentration, complex ions form by reactions such as
Pbl2(.v) + r -» Pblj.
6-23.
(a) BF3
(b) AsF5
6-24.
[SnCI2(a£?)] =
[Sn 2 +][ ci-]2
ß2 => [SnCl2<fl?)] = ß2[Sn2+][Cl-]2 = ( 12)(0.20)(0.20)2 = 0.096 M
Chemical Equilibrium
6-25.
57
[Zn2+] = ATsp/[0H"]2 = 2.« x 10-3 M
[ZnOH+] - p|[Zn2+] [OH"] = ßiA-sp/[OH-] = 9 x 10"« M
[ZníOH)^)] = ß2[Zn2+] [OH]2 = p3ATsp [OH] = 6x lO^M
[Zn(OH)"] = p3[Zn2+] [OH"]3 = ß3A:sp[OH"] = 8 x 10-9 M
[Zn(OH)24] = ß4[Zn2+] [OH7» = ß^sp [OH"]2 = 9 x lfr«4 M
Na+ +
6-26.
Initial concentration:
Final concentration:
O-*) 2
OH"
1
1 —X
^
NaOH(acj)
1
1 -x
0
x
« 0.2 => x =0.15 M
x = 0.2 - 0.4* + 0.2*2
0.2*2-1.4*+ 0.2 = o
x =
-b±ylP-4ac
^
_ 1.4±^1.42-4(0.2X0.2) _
- 6.85 or 0.15
2(fJ 2 )
jc cannot be greater than 1 (the initial or formal concentration of NaOH), so the
correct answer must be 0.15. That is, 15% of the sodium is in the form NaOH(tf<?).
6-27.
Pbl 2 (5)& Pb2+ + 21Pb2+ + 21- ^
Pbl2(aq) ß2 = [Pbl2(a?)] / [Pb2+][I-]2 = 1.4 x 103
Pbl2(i) f* Pbl2(a?)
6-28.
Ksp = [Pb2+][I-]2 = 7.9 x lO"9
K = Kspß2 - 1.1 x lO"5 = [Pbl2(aq)]
Lewis acids and bases are electron pair acceptors and donors, respectively:
+
F3B + :Ö(CH3)2 ->
Lewis Lewis
acid
base
F3B-Ö(CH3)2
. .,
Adduct
Br0nsted acids and bases arc proton donors and acceptors, respectively:
1I2S + ^ 5 N :
-* < 0 N H +
+ HS
Bronsted Branstcd
acid
base
6-29.
(a) An adduct
(b) dative or coordinate covalent
(c) conjugate
(d) [H+] > [OH"]; [OH*] > [H+]
58
Chapter 6
6-30.
Dissolved CO2 from the atmosphere lowers the pH by reacting with water to form
carbonic acid. Water can be distilled under an inert atmosphere to exclude CO2,
or most CO2 can be removed by boiling the distilled water.
6-31.
SO2 in the atmosphere reacts with moisture to make II2SO3, which is a weak acid.
H2SO3 can be oxidized to II2SO4, which is a strong acid.
','
6-32.
H-C-H
H
u
I
I
H-C—N+
I
H
l
"Ö—H
y
C-H
l
There is no place for OH" to
bond to(CH 3 ) 4 N + .
H
H-C-H
I
II
6-33.
(a) HI
6-34.
2H2SO4 ^
6-35.
acid
(a) H 3 0 +
base
H2O
(a) H3NCH2CH2NH3
(b) C6H5CO2H
H3NCH2CH2NH2
C 6 H 5 C0 2
(b) C 5 H 5 NH +
C5H5N
6-36.
(b) H 2 0
HSO4 < II 3 S04
(a) [H+] = 0.010 M => pH = -log [H+] = 2.00
(b) [OH"] = 0.035 M => [H+] = ATw/[OH"] = 2.8 6 x 10"13 M ^ pH = 12.54
(c) [H+] = 0.030 M => pH - L52
(d) [H+] = 3.0 M =5. pH =-0.48
(e) [OH"] = 0.010 M => [H+] = 1.0 x 10-' 2 M => pH = 12.00
6-37.
(a) Kw = [H+] [OH] = LOI x 10-14
X
JC2=1.01X
a t 25"C
X
10-I4 => * = [ H + ] = 1.00s x 10"7M=> pH =-log [H+] = 6.998
(b) At 100°C,pH = 6.132
6-38.
Since [H+] [OH"] =1.0x 10"]4, K = [II+]4 [OH] 4 = 1.0 x 1Q-56
59
Chemical Equilibrium
6-39.
[La3+] [OH-]3 = A;sp = 2 x 10"2 '
[OH"]3 = Ksp I (O.OIO) => [OH"] = 5.8 x 10"7 M => pH = 7.8
6-40.
(a) At 25°C, Kw increases as temperature increases =5> endothermic
(b) At 100oC, Kw increases as temperature increases => endothermic
(c) At 300°C, Kw decreases as temperature increases => exothermic
6-41.
6-42.
See Table 6-2.
RCO2H
Carboxylic
acids
Weak acids:
R3NH+X"
Ammonium
ions
RCO¿M+
R3N:
Weak bases:
Carboxylate
ions
Amines
6-43. CI3CCO2H ^
Mctal
ions
ci 3 cco¿ + H*
<P)-NI.Î^<Q-NH2 + H*
La 3+ + H2O ^
6-44. u 2 ) N
LaOH2+ + H +
2° ^^ C 3 ^ H
+H
HOCH2CH2S- + H 2 0
^
+ 0H
HOCH2CH2SH + OH"
H + + C023"
6-45.
Ka: HC0 3 ^
Kb: HCO3+ H 2 0 r* H 2 C0 3 + OH"
6-46.
(a) H3NCH2CH2NH3
¿
H2NCH2CH2NH3 + H +
H2NCH2CH2NH3
^
H2NCH2CH2NH2 + H+
«»1
(b) "O2CCH2CO2 + H 2 0
H0 2 CCH 2 CO¿ + H2O
6-47.
(a), (c)
6-48.
CN" + H 2 0 ^
^
*b2
^
HCN + OH"
HO2CCH2CO2 + OH
HO2CCH2CO2H
+ OH"
K,
Kb =jjr = 1.6 x 10 5
Chapter 6
jr>"
6-49.
H2PO4 £
HP0 4 " + H +
HC2O4 + H 2 0
6-50.
ATal = ^
= 7.04 x 10-3
Ä'a2
* * - §¡¡¡ -
Kw
*b2~
¿ 2 H2C2O4 + OH
6.25 x lO-8
4.3 x 10-13
6-51.
Add the two reactions and multiply their equilibrium constants to get K = 29x 10"6-
6-52.
(a) Ca(OH)2 (5) =* Ca 2+ + 20H"
jr
2ar
x{2x)2 - tfsp = 10-5.19 => x = 1.2 X lO"2
M
(b) Since some Ca 2+ reacts with OH" to form CaOH+, the A:sp reaction will be
drawn to the right, and the solubility of Ca(OH)2 will be greater than we
would expect just on the basis of K^.
6-53.
Reversing the first reaction and then adding the four reactions gives
Ca 2+ + C0 2 (g) + H 2 0(/) =* CaC03(5) + 2H+
K =
KQQ2K\K2IKSP
K = (3.4 x 10-2X4.5 x 10"7X4.7 x l()-ll)/(6.0 x 10"9) = 1.2 x lO'lO
[HI2
[C^]Pco2
_ (1.8» 10-7)2 _
- [Ca2+][0.10]
K
-
12X
[Ca2+] = 2.7 x 10-3 M - 0.22 g/2.00 L
,(H
CHAPTER 7
ACTIVITY AND SYSTEMATIC TREATMENT OF EQUILIBRIUM
7-1.
As ionic strength increases, the charges of the ionic atmospheres increase and the
net ionic attractions decrease. There is less tendency for ions to bind to each other.
7-2.
(a) true
7-3.
(a) {[0.008 7 • l 2 + 0.008 7 • (-1) 2 ] = 0.008 7 M
(b) true
(c) true
(b) { [0.000 2 - 3 2 + 0.000 6 -(-l) 2 ] - 0.001 2 M
(b) 0.54
(c) 0.18 (Eu 3 + is a lanthanide ion) (d) 0.83
7-4.
(a) 0.660
7-5.
The ionic strength 0.030 M is halfway between the values 0.01 and 0.05 M.
Therefore, the activity coefficient will be halfway between the tabulated values:
y = ¿(0.914 + 0.86) - O.887.
7-6.
(a) !
logy
W
0.51-22^/0083 =
1 + (60(^/0.083 / 305)
^
J
'
J
0375
=
^ <
*>
(b) y= (Q0Of_~00Q55) (0-405 - 0.485) + 0.485 = 0.432
7 7
T = (0Ó0l3-~0°055) ( 0 1 8 • 0 2 4 5 )
7-8.
1 f [ether(ai/)] becomes smaller, ycther must become larger, since
" '
+
°245
=
°'2°2
K{= [ethcr(o(7)] Y ether ) is a constant.
7-9.
The solubility of Hg2Br2 is small, so we assume that Hg2Br2 contributes negligible
Br to 0.001 00 M KBr.
p - 0.001 00 M, [Br] = 0.001 00 M, yH
2+
= 0.867, y ßr - = 0.964
+L
J
Ksp = 5.6 x 10-23 = [Hg 2 2 + ]'YHa2
H g ¿ f[Br-]2y¿'Br
2
= [Hg22+j](0.867)(0.001
^ . o o / ^ u . u u i 00)
u0) 2(0.964)2
(0.964) 2
=> [Hg22+] =7.0 x 10-17
M
Check our assumption: Yes, B r from Hg2Br2 is negligible.
7-10.
The solubility of Ba(103)2 is small, so we assume that Ba( 103)2 contributes
negligible IO3" to 0.100 M (CH3) 4 NI0 3 .
61
62
Chapter 7
p =0.100 M, [I03_] = 0.100 M, 7^2+ =0.38, y| O -=0.775
1.5x10-9 = l B a 2 , ] y B i i 2 + [ I 0 3 - ] 2 y ^
Ksp=
= [Ba2+](0.38) (0.100)2 (0.775)2
7-11.
Ionic strength =0.010 M (from HCl)+ 0.040 M (from KCIO4 that gives
K+ + CIÛ4) = 0.050 M.
+
Using Table 7-1, Y,,+ - 0.86.
pH =-log[H ]Y H + =
7-12.
=> [Ba 2+ ] - 6.6 x 10 7 M
log[(0.010X0.86)] = 2.07.
Ionic strength = 0.010 M from NaOH + 0.012 M from LÍNO3 = 0.022 M.
Interpolating in Table 7-1 gives Y OH - = 0.873.
Kw
1 n x 10 1 4
+
= 1.15 x 10-12
[H ]YH+ = [OIF]YOH(0.010X0.873)
pH = -log(1.15x 10-12) = 11.94
If we had neglected activities, pH = - log[H+] = -log
7-13.
[OH"]
= 12.00
e = 79.755 e-*-* * >°"3 (323.15 - 293.15) = 69.474
logy =
(-1.825 • UK>)|(69.474) (323.15)1 3 / 2 (-2) 2 yfOAQQ
400^0.100
1+
2.00V(69.474)(323.15)
= -0.482 6 => y = 0.329
7-14.
K
(In the table, y - 0.355 at 25°C.)
Spreadsheet for Debye-Hückel calculations
1
2
3
4
5
6
7
8
9
10
11
12
13
A
li
C
D
E
Ionic strength
Gamma (z - i i ) Gamma (/ -;> Gamma {/ - ±3) Gamma (z = ±4)
0.0001
0.988
0.955
0.901
0.831
0.980
0.0003
0.924
0.836
0.727
0.001
0.965
0.X67
0.72?
0.565
0.003
0.942
0.583
0.787
0.383
11.ill
0.901
0.660
0.393
0.190
0.03
Ü.847
0.515
0.225
0.071
0.1
0.769
0.350
0.094
0.015
H2
C2 =
D2 =
li2 =
10 (0.5l*Sqrt(A2)/(l+400«Sqrt(A2V305))
l0 A (-0.51 *4*Sqrt(A2)/( 1 +400*Sqrt(A2)/305))
ia A (-O.51*9*Sqrt(A2)/(l+400*Sqr1(A2V305))
10 A (-0.5l*16*Sqrt(A2)/(l+400»Sqrt(A2)/305))
63
Activity and Systematic Treatment of Equilibrium
S=
t
0.6
.001
.01
Ionic strength (M)
.0001
7-15.
The equilibrium constant is
HA
H+ + A-
Ka =
[H+]yH- [A-JYA-
[HA]yHA
*
From the table of activity coefficients, yn+ = 0.83 and yA- • 0.80 at p - 0.1 M.
For HA, we estimate
logYHA = *p = (0.2X0.1) - 0.02,oryHA " 10 002 = L05.
Putting activity coefficients for p = 0.1 M into the equilibrium expression gives
_
Ka
[H+]yn+ [A]yA- ' [HA]yHA
rH+1(0.83)[A-](0.80) _ m+ÏÏA1
[HA](1.05)
" U 0 J [HA]
ÜQA-1,.,
[HA] (p = 0.1 M) = AyO.63.
The activity coefficients at p = 0 are all 1, so the equilibrium expression is
or
_
[H+]yH+ [A-]YAK
*~~
[HA]y„A
[H+1(n[A-l(l)
[HA](1)
\B+]\A-]
[HA] &
^
The concentration quotient at the two different ionic strengths is
ÎH-ÏÏA-1
Concentration quotient =
iHAL
(ji = 0)
™ < P = 0,M>
K»
= 0.63
AyO.63
in agreement with the observed value of 0.63 ± 0.03.
7-16.
The charge balance states the magnitude of positive charge equals the magnitude of
negative charge in a solution. That is, the solution must be neutral. The mass
balance states that atoms are conserved. If we deliver a certain number of atom A
into a solution, then the sum of atom A in all species must equal the atoms of A
64
Chapter 7
delivered to the solution. If we deliver a certain ratio of atoms A and B into the
solution, then the sum of atoms of A and B in all species must be in that same ratio.
7-17.
Charge and mass arc proportional to molarity, not to activity.
7-18.
[H+] + 2 [Ca2+] + [Ca(HC03)*] + [Ca(OH)+] + [K+] =
[OH"] + [HOO3] + 2[C023"] + [CIO4]
7-19.
Charge: [H+] = [OH"] + [HSO4] + 2[S024"]
7-20.
+
2
[H ] - [OH"] + [H2ASO4] + 2[HAs0 "] +
3[As034"]
p
H • O - Ás- O"
Ô"
7-21.
2
+
+
(a) Charge balance: 2[Mg ~] + [IF] + [MgBr ] + [MgOH ] - [Br"] + [OH"]
Mass balance: total Br = 2(total Mg)
[MgBr+] + [Br"] = 2{[Mg2+] + [MgBr+] + [MgOH+]}
(b) [Mg2+] + [MgBr+] + [MgOH+] = 0.2 M
[MgBr+] + [Br"] = 0.4 M
7-22.
250 mL of 1.0 x 10"6 M charge = 0.25 x 10 6 moles of charge.
(0.25 x 10-6 m o l e s of charge) (9.648 x l f ^ _ ^ 2 ^ s _ j = 0 0 2 4 1 2 C
The dielectric constant of air is e = 1 and the separation is 1.5 m.
Horco - -(8.988 * |0>) ^ ' ¡ g f f i
2 4
' 2 ) - 2.3 K ,O> N.
(2.3 x 106 N) (0.224 8 pounds/N) = 5.2 x 105 pounds.
Two elephants do not weigh enough to keep the beakers apart.
7-23.
[CH3CO¿] + [CH3CO2H] = 0.1 M
7-24.
Ytola[ = jXtotal
2[X2Y2+]+[X2Y4+]+3[X2Y3]+[Y2-] = f {2[X2Y2+]+2[X2Y4+]+2[X2Y3]}
[Y2-] = [X2Y2+] + 2[X2Y4+]
7-25.
3 (total Fc) = 2 (total sulfur)
3{[Fe3+] + [Fe(OH)2+] + [Fe(OH)J] + 2[Fe2(Of l)42' J <• [FcSOj]}
= 2{[FeSOj] + [S024] + [HSO¿]}
We write 2 in front of [Fe2(OH)2+] because Fe2(OH)2+ contains 2 Fe.
65
Activity and Systematic Treatment of Equilibrium
7-26.
(a) Pertinent reactions:
ft
[HA]YHA[OH-)YOH-
A" + H 2 0 ^ HA + OH"
Kb = —
= 5.7x10-'°
(A)
H 2 0 S H+ + OH"
14
Kw = [H+]YH+ [OH-hoH" = 1.0 x io-
(B)
[A-]yA-
Charge balance: [H+] + [Na+] = [OH'] + [A ]
Mass balance: [Na+] = 0.01 M • F
Mass balance: [HA] + [A] = 0.01 M s F
(C)
(D)
(E)
(b) Now we neglect activity coefficients. We will make the following
substitutions in the charge balance:
[OIF] = KJ[H']
From Equation A: [HA] = T^j"^
(F)
(G)
From Equation E: [HA] = F - [A]
Now equate the expressions for [HA] from Equations F and G to solve for [A"]
* ^ = F-1A-1
[OH-]
' lAJ
KoV0
=F
^^-T^n
Now substitute KJ[ti*] for each [OH] in the equation above to get
F/fjrH'1 _
F*.
rAl
A
+
t J - Kh + KJ[H ] - tfb[H+] + K„
We can now substitute for all terms in the charge balance using [A*] from
Equation H, [OH] = ÂVÎH*], and [Na*] - F:
Charge balance: [H+] + [Na+] = [OH"] + [A-]
w+*
= m+Kb[H*w+Kw
m
Kn}
®
+
Equation I has the form we were looking for. The only unknown is [H ]. For
convenience infindinga numerical solution, 1 willrearrangeas follows:
° = # T rfofc- <H+i-F
<J)
The following spreadsheet evaluates the right side of Equation J in cell D9.
Guess a value for [H+] in cell B7. Before using Goal Seek in Excel 2007, click
the Microsoft Office button at the top left of the spreadsheet, click on Excel
Options, and then on Formulas. Set Maximum Change to le-14. In earlier
versions of Excel, go to Tools and Options and select the Calculations tab and
set Maximum change to le-14. Then execute Goal Seek to vary [H+] in cell
B7 until the sum in cell D9 is close to 0 (within le-14). The answer in cell B7
is [H*] = 4.19 x 10"9 M or pH = 8.38.
66
Chapter 7
Cells C15:C17 compute other concentrations from the relations
K„
l'A',
m
A1 _
[OH] =
[HA]
- ^ffA]
[A"] = K*[\r] + K*
[OHr]
[H+]
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
A
B
C
Finding the species in NaOAc solution
Using GOAL SEEK to Solve for [H*]
K „ = 1.00E-14
K b = 5.70E-10
F=
0.01
Guess for [H*] =
4.19E-09
F
IpH = -k>g[H*] =
K j p - n + F M K b [ H ' ] + K J -[rF] - F =
T
8.38
1.41E-16
I
I
Tools - > Options --> Calculation ~> Maximum change = 1e-14
Use GOAL SEEK to vary [H*] in cell B7 until the sum in
cell D9 is equal to zero
[OHl = M H t } =
16 [A] = FK,/(K b [H t ]+K w )=
17
[HA] = Kt,[AT/[OH] =
7-27.
D
2.39E-06
1.00E-02
2.39E-06
(a) Pertinent reactions:
K
Ca(OH)2(s) ^ w Ca 2+ + 20HCa
2+
ft
+ 01F ^ CaOH
+
Ksp = [Ca2+]YCa24[OH-]2y 2 . = 6.5 x 10-6
K\ =
[CaOH+]YCaOH+
[Ca2+]Yca2+[OH]y
- 2.0 x loi
OH"
H20 ^
+
H + OH
*w = [H + ]y H+ [OH-]y OH - = 1.0 x 10-14
Charge balance: 2[Ca2+] + [CaOH+] + [H+] = [OH"]
Mass balance: [OH] + [CaOH+] = 2{[Ca2+] + [CaOH+]} + [H+]
species containing OH"
species containing Ca 2 +
(Mass balance gives the same result as charge balance.)
Then arc 4 equations (3 equilibria and charge balance) and 4 unknowns:
[Ca2+], [CaOH+], [H+], and [OH],
(b) The approximations we make are to disregard the activity coefficients and to
neglect [H+] in the charge balance because [H+] « [OH"] in basic solution.
The charge balance becomes
67
Activity and Systematic Treatment of Equilibrium
2[Ca2+] + [CaOH*] = [OH']
(A)
Substituting [CaOH+] = Aj [Ca2 ' ][OI I] into (A) gives
roH-i
[Ca2+]
2[Ca2+] + A:i[Ca2f][OH-] = [OH-]
2 + K\ [OH]
Substitute this expression for [Ca2+] into the solubility product:
ÍQH-13
Ksp
= [Ca2+][OH-]2 =
L
*P " lv - a_ J l v " '
2 + K\[OH]
(H)
We solve Equation B in the spreadsheet by guessing [OIF] in cell C4 until the
expression in cell D4 is equal to Ksp. Wc used Goal Seek for this purpose.
D
A
B |
C
1 Ca(OH) 2 solubility
2
[OH"]9UBSS =
3 K«p =
4
6.5E-06
5 K,=
[OH f /(2 + K,[OH']) =
0.0253528
6.5000E-06
[CaOhf] =
6
2.0E+01
[Ca 2 *] =
0.0051276
7
0.0101126
8
A
[H*] = MOM"] =
9 D4 = C4 3/(2 + A6*C4)
A
3.94E-13
10 C7 = A4/01 2
11 D7 =A6*C7'*C4
[CaOH+] = 0.005 I M
Results: [Ca 2+ ] = 0.010 I M
[OH-] = 0.025 4 M
[H+] = A: W /[OH-] = 3.9 x 10-»3 M
Total dissolved Ca = 0.010 1 + 0.005 1 = 0.015 2 M
The formula mass of Ca(OH)2 is 74.09 g/mol, so 0.015 2 M is 1.1 j g/L. The
Handbook of Chemistry and Physics lists the solubility of Ca(OH)2 as 1.85 g/L
atO°CandO.77g/Latl00°C.
7-28.
(a) Pertinent reactions:
Zn 2+ + SO*2t ^
ZnS0 4 (a?) K\10npa,r
[ZnS04(i/</)]
= 2.2x 102
2
"[Zn + ]Y Zn 2 + [S0 4 ]y S0 2 4 2
(y7nSQ = 1 because the ion pair is neutral)
H 2 0 ?± H+ + OH
*w = [H + ]y H +[OH]y OH - = 1.0 x 10-'4
Charge balance: 2[Zn 2+ ] + [H+] = 2[S0 2 4 ] » [Olli
Mass balance:
0.010 M = [Zn2+] + [ZnS04(««/)]
0.010 M = [SO4] + [ZnS0 4 (u^)]
5 equations and 5 unknowns: [Zn 2+ ], [S024"], [ZnS04(«<?)], [IF], [OIF]
68
Chapter 7
Since there is no coupling of the zinc sulfate and water equilibria, [H+] = [OH]
= 1.0 x lO-7 M and we can disregard H + and OH" in this problem.
Substituting [ZnS04(i7<7)] = 0.010 - [Zn 2+ ] and [SO^ ] - [Zn 2+ ] into the ion
pair equilibrium expression gives
K.
Ensoul
„„2~,
K|onpair _ ir , * , ,
[Zn2+]yZn2+[S024}yS024r
¿,¿ x K)*-
0.010-[Zn 2 + ]
^ionpair .^ , . ,
__ 0 + n
- 2.2 x | 0 2
[Zn2+]yZn2+[Zn2+]yS02-
Rcairanging gives
(220)[Zn2+]2yZn2+yso2- + [Zn2+] - 0.010 = 0.
(A)
Setting yZn2+ = Yso2,-= 1 allows us to solve quadratic Equation (A) to find
[Zn2+] = 4.8 4 x lO"3 M.
(b) Ionic strength = |([Zn 2+ ] • 4 + [S04"] • 4) - 0.0194 M
Interpolation in Tabic 7-1 gives y7n2+ — 0.630 and y s o 2- - 0.609.
Putting these values of y into Equation (A) gives [Zn 2+ ] = 6.47 x 10"3 M
3 r d iteration: p =4(6.4 7 x 10"3) = 0.025c M
yZn2+ = 0.599 yso24- = 0.574
[Zn2+] = 6.65 x 10"3 M
4 [h iteration: p = 0.0266 M
yZn2+ = 0.596
yso24- - 0.571
[Zn2+] = 6.67 x |<H M
O.OIO-6.67 * 10"3 x
Ion-paired percent =
fTÖTÖ"
^
=
^%
Ionic strength = 4 (6.67 x 10"3) = 0.027 M
(c) Acid hydrolysis: Zn 2+ + H 2 0
==
Base hydrolysis: SO4 + H 2 0
=-
ZnOH+ + H +
JfcV
HSO¿ + OH"
Appendix I gives the formation constant iß\) for ZnOH+.
Wc can find Ka for Zn by adding the ß\ reaction to the Kw reaction:
Zn 2+ + OH
U
ZnOH+
H2O
==
H+ + OH-
Zn2+ + H 2 0
^
log/?, = 5.0 => p\ = 10 50
Kw=\0-l4M
ZnOH+ + H+ K, = ftKw= 10"9'0
69
Activity and Systematic Treatment of Equilibrium
2_
The base hydrolysis constant for SO 4 is KJK^ for I FS( ).(:
SO4 + H'
7-29.
' ^
H2O
&
SO 4 + H 2 0
^
\/Ka2= l/10-'-w
HSO¿
H+ + OH"
K W =10 , 4 Ü Ü
HSO; + OH"
Kh = KJK92= 10*1"1
Pertinent reactions:
LiF(s) Ç= Li* + F
Ksp = [Li + )y L i + [r]y F - = 0.0017
LiF(s) ¥= UP(aq)
KionpaiT = [LiF(aq)]yUF = 0.002 9
H 2 0 & H+ + OH
Kw - [H + ]y H+ [OFT\yOH- - 1.0 * 10 1 4
Charge balance: [Li+] + [H+] = [F ] + [OIF]
Mass balance: [Li+] + [LiF(o<?)] = [F] + [LiF(aq)]
There are five equations and five unknowns: [Li + ], [F~], [LiF(a^)], [H+], and
[OH ]. There is no coupling between the LiF reactions and the dissociation of
water, so [H+] = [OH~l = 1 x 10"7 M. [H+] and [OH~] cancel in the charge balance,
leaving [Li+] = [F~]. Also, the ion-pairing equilibrium constant tells us that
[LiF(flfl)] = 0.002 9 M with the good approximation that yyp = 1 . All that is left is
to find [Li+] and [F ] from the solubility product with the condition [Li+] = [F"].
[Li+]yLj+ [F"]YF- = *sp
[Li+]2yL.+ yF- - Ksp
=> [Li+] = [F] =
1s" iteration: Let yL¡+ = y p = 1
2 nd iteration: p = 0.041 M
^
^
=> [Li+] - [F ] = -sßTp = 0.041 M
=> y y + = 0.843 and y p = 0.824
=> [Li+] = [F] = ^risp/Yj j+ Y F . = 0.049 M
3 r d iteration: p = 0.049 M
=> y u + = 0.834 and y p = 0.812
=> [Li+] = [F] = ^/C sp /y L .+ yF- = 0.050 M
4 th iteration: p. = 0.049 M
=> y u + - 0.833 and y p = 0.811
=> [Li+] = [F] = ^ s p / Y L i + Y F - = 0.050 M
70
7-30.
Chapter 7
(a)
CaC03(.ï)
C02(aq) + H 2 0
CO2." + H+
^
Ca 2+ + CO^"
Ksp - 4 . 5 x 1 0 9
^
HCOj + H +
K\
^
HCO5
\/K2= 1/(4.69x10-1')
CaC0 3 (i) + C02(aq) + H 2 0 ^
Ca 2+ + 2HCOj
= 4.46x 10*7
K =
KipK\lK2
= 4.2 8 x lO"5
(b) The equilibrium constant for the net reaction is
[Ca2+][HCO$]2
[C0 2 ( w / ) ]
=^=4.28xl0-5
We can substitute into this equation [HCO3 ] = 2[Ca2+] and [C02(aq)\ =
*C0 2 A:02 (where KQç^ = 0.032 and P C 0 2 = 3 8 x 10"4 bar) to get
rCa2+¥2rCa2+1^2
A- C 0 ? P C 0 2
= K
=>
[Ca2+] = 5
° 7 * 10"4 M
= 2
°
mg/L
(c) If [Ca2+] = 80 mg/L = 2.0 x 10-3 M, then
Pc
°2
-
ÍCj2+j(2[Ca^r¿
KC02K
- 0-023 ba.
The partial pressure of CO2 in the river is about (0.023 bar)/(3.8 x 10-4 bar) =
60 times higher than the atmospheric pressure of CO2. There must be a source
of extra CO2 such as respiration in the river or inflow of ground water that is
very rich in CO2 and not in equilibrium with the atmosphere.
CHAPTER 8
MOINOPROTIC ACID-BASE EQUILIBRIA
8-1.
HBr (or any other acid or base) drives the reaction H 2 0 ^ H+ + OIF to the left,
according to Le Châtelier's principle. If, for example, the solution contains I0"4 M
HBr, the concentration of OH - from H2O is KW/[W] = 1 0 1 0 M. The concentration
of H+ from H2O must also be 1 0 ' ° M, since H+ and OH" arc created in equimolar
quantities.
8-2.
(a) pli = -log[H+] = -log( 1.0 x 10*3) = 3.00
(b) [H+] = K w /[OH-] - (1.0 x I0 1 4 )/(l.0x lO"2) = 1.0 x 10-'2 M
pH = -log [IF] = 12.00
8-3.
Charge balance: [II ' j - [OIF] + [CIO4 ] => [OH'] = [H+] - 5.0 x 10"8
Mass balance is the same as charge balance.
Equilibrium: [H+] [OH'] = Kw
[H + ]([H + ]-5.0x I0-8) = 1.0 x lO« 4 => [H+] = 1.28 x 10 7 M
pH = -log [H+] - 6.89
[OH"] = Kwl[H+] - 7,8 x 10-8 M => [H+] from H 2 0 = 7.8 x 10"8 M
7.8 x 1 0 8 M
+
Fraction of total [H ] from H2O = 1 28 x lO*7 M = ° ' 6 1
8-4.
(a)
pll = -log[H + ]y„+
1.092 =
log (0.100) yH+ => y„+ = 0.809
The tabulated activity coefficient is 0.83.
(b) 2.102 - -log(0.0100)y n + => yH+ - 0.791
(c) The activity coefficient depends somewhat on the identity of the counterions in
solution.
»-S-
<7^-C02H
(b)
/ 0 ) - C O 2 + H2O -
/QVCO 2 H + OH-
(c)
/QVNH
2
^
/ Q V N I I , 1 Oil
(d)
<^-NH
3
¡±
^)^NH
+ H20
Ç=
< ^ Ç ^ C 0 2 + H+
(a)
71
2
+ H+
Ka
Kb
Kb
Ka
72
8-6.
Chapter 8
Letjc=[H + ] = [A"] and 0.100 -x = [HA].
JC 2
- 1.00 x 10-5 =>* = 9.95 x 10-4 M => pH = -log* = 3.00
Q1QQ_Y
[A'l
a =
8-7.
9.95 x 10-4
=9 95Xl
=
ÎAT^ ^ÏOÔ-
BIP
0.100 -x
^
B + H+
x
x
-
°-3
Ka = KjKb
= 1.00 x 10-10
X2
0.100-x = LOO x lO-"» ^ x = [B] = [H+]= 3.16 x 10-6 M => pH = 5.50
8-8.
(CH 3 ) 3 NH + ^
F-x
(CH3)3N + H +
x x
Ka = 1.59 x 10"'0
x2
0.060 -x = ^a => ^ = 3.O9 x 10-6 => pH = 5.51
[(CH3)3N] = x = 3.1 x 10-6 M , [(CH3)3NH+] = F - j c = 0.060 M
K&
8-9.
HA ^
H
+
rAi[H + ]
K A". Q = r | |' A , '. When the system is at equlibrium, Q = Ka.
Let's call the concentrations at equilibrium [A-]e, [H + ] c , and [HA]e. If the solution
is diluted by a factor of 2, the concentrations become 5 [A"]c, 2 [H + ] e , and 2 [HA]e.
2[A"]e2"[H+]e
1 [A-]e[H+]c
|
The reaction quotient becomes Q = — i
= 7—iTTÂï— = T ^al AJc
f[HA] e
"
Since Q < Ka, the concentrations of products must increase and the concentration of
reactant must decrease to attain equilibrium. That means that the weak acid
dissociates further as it is diluted in order to stay in equilibrium.
M0.
HA 5 H* + AI
r
X
„
F o r F =
X
X
K
*
x2
W§
io_Jf
ForF=10Ka,
K . -[HA]
™W
" F-x
= K
[0^_X
x
0.092 ÂTa
* =* * = 0-092K a ; a = p = 0A00K&
- 92%
= K& => x = 2JKa;
ct=f = " [ ^
= 27%
CO 99 F)2
For99%dissociation,x = 0.99F => Ka = p J 0 9 9 F => F = (0.0102)tfa
73
Monuprolic Acid-Base Equilibria
] . . 10-2.78
10-2.78
10-2.78
nO-2.78\2
K
> • 0.045 0 - lO-»«
8-12.
HA Ç* H+ +
A"
F-x
x
x
= 6M
* '°-5
=
>*•
=
4 2
°
a = 0.0060 = |
F = 0.045 0 M and x = (0.006 0)(0.045 0 M) = 2.7 x 10^ M
x2
=> Ka = F ^ = L 6 * l 0
=* pK* = 5 J 9
8-13.
H + + A"
x
x
2
2
x
„
x
= K* => n m n ,- = 9.8 x 10"5 => x = 9.4 x 10 4
F-JC " A a w 0.010-.v
(a)
HA
F-x
^
=> pH = 3.03
a - — ^ — = fF = 9.4%
[HA] + [A-|
(b) pH = 7.00 because the acid is so dilute. From the Ka equilibrium we write
Kâ
9.8 * 10"5
[A"] = ¡ ^ [ H A ] = ]0y]0.1
[HA] = 980 [HA]
„ ..
TA-1
980 [HA]
980 _
' [HA] + [A-] " [HA]+ 980 [HA] " 981
8-14.
"**
Phenol is a weak acid, so it will contribute negligible ionic strength. The ionic
strength of the solution is 0.050 M.
HA
F* H+ + A"
F-x
x
x
[H+]YH'[A-]YA-
[HA]y | | A
~K*
Ka = 1.01 x 10"»0
(x)(0.86Xx)(0.835) =
(0.0500-x){ 1.00)
,UI
lU
Q
pH - -log[H+]yH+ = -log[2.65 x 10-fi](0.86) = 5.64
a =
^
= 2 n 5 n<r!n^ = 5.30 x 10-* = 0.005 3%
0050
[HA] + [AJ
°
=
=* *
¿A>:>
1U
6
74
8-15.
Chapter 8
£a I
Cr 3+ + H 2 0
0.010-x
Cr(OH)2+ + H+
— = 10-366 => x = i.3 7 x 10-3 M
0.010-Jc
pH = -log x = 2.86
8-16.
HN0 3
F-x
F-JC
= 26.8
o.
H+ +
0.010
0.14
NO;
x = 0.099 6 M when F = 0.100 M => a = p = 99.6%
x = 0.965 M when F = 1.00 M = > a = ^ = 96.5%
8-17.
The initial spreadsheet follows (on the left). Guess a value for* in cell A4. The
formula in cell B4 is "=A4A2/(A6-A4)'\ In Excel 2007, click the Microsoft Office
button at the top left of the spreadsheet, click on Excel Options, and then on
Formulas. Set Maximum Change to a small number such as le-20 to find an
answer with high precision. In earlier versions of Excel, in the Tools menu, go to
Options and then Calculation. Check Iteration and set Maximum change to le-20.
Highlight cell B4 and select Goal Seek. Set cell B4 To value le-5 By changing cell
A4. Click OK and Goal Seek finds the solution in the second spreadsheet (on the
right). The value x = 9.95 x 10 5 makes the quotient x2/(F-x) equal to 1.00 x 10"5.
A
B
1 Using Excel GOAL SEEK
2
3 X =
xV(F-x) =
4
0.01 1.1111E-03
5 F=
6
0.1
A
B
1 Using Excel GOAL SEEK
2
3 x=
xz/(F-x) =
4
0.00099501 1.000OE-O5
5 F=
6
0.1
Before executing Goal Seek
After executing Goal Seek
8-18.
The "fishy" smell comes from volatile amines (RNH2). Lemon juice protonates the
amines, giving much less volatile ammonium ions (RNH3 ).
8-19.
Letjc = [OH"] - [BH+] and 0.100 -x = [B].
Q ^ . ^
= 1.00 x 10-5
B
x = 9.95x 1(HM =>[H + ]= - f =1.005 x 10- n =>pH = 11.00
=
[BH4]
[B] + [B1F]
9.95 x IP"4
- 9.95 x 10-3
0.100
Monoprotic Acid-Base Equilibria
8-20.
(CH 3 ) 3 NH + + OH"
x
x
(CH3)3N + H 2 0 ^
F-x
2
= Kb^
QüIQ_X
x = 1.9, x JO-3 ^
Kb - W ^ a - 6.3 x \0-5
K
pH = - l o g - f = 11.28
[(CH3)3NH+] = JC = 1.9j x 10-3 M , [(CH3)3N] = F-x
8-21.
CN" + H 2 0 F* HCN + OH"
F-x
x x
Kb - Kw/K9 - 1.6 x 10-5
2
/Í
0.050-JC
8-22.
= 0.058 M
=
K
*> => * = 8.9 x i 0 " 4 => P H = - , 0 8 " T
=
10 95
-
CH3CO2 + H 2 0 T¿ CH 3 C0 2 H + OH" Kb = ff«/** = 5.7 x 10-10
F -x
v
x
(1.00xl'ai)-, - * » * - ™* 1H =» « = f -
MOT«*
OJO.Io^-x - * * * - " « » • » • - ! - " M «
For 1.00 x 10-12 M sodium acetate, pH = 7.00 and we can say
*b[A"]
[HA] = - 5 L - i = (5.7 x 10-3)[A"]
[OH"]
a
= - £ £ - =
[HA] + [A-1
(5.7xlO-3 )fA -1
(5.7 x 10-3)[A"] + [A"]
The more dilute the solution, the greater is a.
8-23.
B
+
9 28
F-(AVIO" - )
H20
(Kw/10-928)2
*
b
BH+
+ OH"
KJW-W
Jíw/10-9-28
(/Cw/10-9.28)2
J ô x
" F - ( / C W / I O - 9 2«) " O.IO-(A: W /IO-9-28) -
8-24.
B + H2O T= BH+ + OH" a = 0.020 = |
0.10-x
x
(2.0x10-3)2
" 0.10-JC " 0.10 (2.0 - 10-3) - «•'
«
,u
As [B] -> 0, pH -» 7 and [OH"] — 10"7 M.
a
=
[BH+]
[B] + [BH+]
=
10 7 /f b [B]
[B] + lO7 Kb [B]
=
,W
"
=> x = 2.0 x 10 3 M
x
Xl
K
Ab
8-25.
F*
10 7 *b
1 + lO7 Kb
76
Chapter R
WKb
For Kb = 10- , we have a = y ^ j ^
4
ÎO7^-4
= , + 10 7 , 0 -4 = 0-999
l07Kh
For Kb = 10-10, w e
have a
=
1+ 1Q7
^ = ,+
io 7 io-'°
l o 7 1Q . 10
= 0.000 999
8-26.
I would weigh out 0.0200 mol of acetic acid (= 1.201 g) and place it in a beaker
with -75 mL of water. While monitoring the pH with a pi I electrode, I would add
3 M NaOH (~4 mL is required) until the pH is exactly 5.00. I would then pour the
solution into a 100 mL volumetric flask and wash the beaker several times with a
few milliliters of distilled water. Each washing would be added to the volumetric
flask, to ensure quantitative transfer from the beaker to the flask. After swirling the
volumetric flask to mix the solution, 1 would carefully add water up to the 100 mL
mark, insert the cap, and invert 20 times to ensure complete mixing,
8-27.
The pH of a buffer depends on the ratio of the concentrations of HA and A"
(pH - pK3 + log [A']/[HA]). When the volume of solution is changed, both
concentrations arc affected equally and their ratio docs not change.
8-28.
Buffer capacity measures the ability to maintain the original [A"]/[HA] ratio when
acid or base is added. A more concentrated buffer has more A" and HA, so a
smaller fraction of A" or HA is consumed by added acid or base. Therefore, there
is a smaller change in the ratio [A"]/[HA].
8-29.
At very low or very high pH, there is so much acid or base in the solution already
that small additions of acid or base will hardly have any effect. At low pH, the
buffer is H 3 0 + /l l 2 0; and at high pH, the buffer is H 2 0/OH\
8-30.
When pH = pKa, the ratio of concentrations [A"]/[HA] is unity. A given increment
of added acid or base has the least effect on the ratio [A"]/[HA] when the
concentrations of A" and HA arc initially equal.
8-31.
The Hcndcrson-I lassclbalch is just a rearranged form of the Ka equilibrium
expression, which is always true When we make the approximation that [HA] and
[A"] arc unchanged from what we added, we are neglecting acid dissociation and
base hydrolysis, which can change the concentrations in dilute solutions of
moderately strong acids or bases.
77
Monoprotic Acid-Base Equilibria
8-32.
add
hydrogen peroxide
propanoic acid
cyanoacctic acid
4-aminobcnzcncsulfonic acid
pA'a
11.65
4.87
2.47
3.23
must suitable because pA'a
is closest to desired pll
8-33.
pH = pKa + log f j ^ j = 5.00 + log ö^Ö" = 4 - 7 0
8-34.
[HCO¿]
pH - 3.744 + log JücÖtf]
pH:
[HC0 2 "]/[HC0 2 H]:
3.000
3.744
LOO
0.180
4.000
1.80
[Hco;]
8-35.
pH = 3.57 + log r H C Q 2 H ] where 3.57 is pKa at p = 0.1 M
8-36.
Substituting pH = 3.744
[HC02"]/[HC02H] = 1.5
[N02gives
]
pH = pKa + log M [ N o 2 ] , where pKa = 14.00-p^b = 3.15
(a) IfpH - 2.00, [HNO2HNO2] = 14
(b) IfpH = 10.00, [HN02]/[NO¿] = 1.4X 10-7
8-37.
(a) HEPES is an acid with pKa = 7.56. When it is dissolved in water, the solution
will be acidic and will require NaOH to raise the pH to 7.45.
(b) 1. Weigh out (0.250 L)(0.050 0 M) = 0.012 5 mol of HEPES and dissolve
in -200 mL.
2. Adjust the pH to 7.45 with NaOH.
3. Dilute to 250 mL,
8-38.
213 mL of 0.00666 M 2,2,-bipyridinc = 1.41a mmol base. We will add JC mol H* to
get a pH of 4.19.
2,2'-bipyridinc +
Initial mmol: 1.419
Final mmol:
1.419 x
HH
2,2'-bipyridineH'
78
Chapter 8
[bipyridinc]
fbipyridincH+]
I.4I9-JC
4.19 = 4.34 + log
=> x = 0.831 mmol
pH = pK& + log
0.831 mmol
volume =
8-39.
(a)
0246mmol/mL
.,
„ M
= 3.38 ml.
+H2
+
(3 ° * o
H
+
(J
+ O
H
"*
H
* f) * H*
N
N
H
H
(b) FM of imidazole = 68.08,
FM of imidazole hydrochloride = 104.54.
„
,
1.00/68.08
„,„
CÄM
pH = 6.993 + log , 0 0 / ,04,54 = 7 1 8
(c)
Initial mmol:
Final mmol:
B
I4.69
12.23
+
pH = 6.993 + log T ^
H+
2.46
->
BH+
9.57
12.03
= 7.00
(d) The imidazole must be half neutralized to obtain pH = pK3. Since there are
14.69 mn
mmol of imidazole, this will require 2(14.69) = 7.34 mmol of HCIO4 =
6.86 mL.
8-40.
(a) pH = 2.865 + log ^ 5 ^
= 2.56
(b) Using Eqns. (8-20) and (8-21), and neglecting [OIL], we can write
=> pH = 2.61
(c) 0.080 mol of HN0 3 + 0.080 mol of Ca(OH)2 react completely, leaving an
excess of 0.080 mol of OH". This much OH" converts 0.080 mol of
CICH2CO2H into 0.080 mol of CICH2COJ . The final concentrations are
[ClCH2CO¿ ] = 0.020 + 0.080 = 0.100 M and [C1CH2C02H] = 0.180 - 0.080
= 0.100 M. SopH = pKa = 2.86.
79
MoTioprolic Acid-Base Equilibria
HA
8-41.
Initial moles:
0.0224
Final moles:
0.022 4-JC
A-
OH"
+
8-42.
0.010 17 mol
0.626 M
H20
x
pi I = 7,40 = pKa + log j j f t j - 7.48 + log 0 0 2 2 V JC
volume =
+
JC =
0.010 17 mol.
16.2 mL
(a) Since pKa for acetic acid is 4.756, we expect the solution to be acidic and will
ignore [OH"] in comparison to [H + ].
[HA] = 0.002 0 - [H+]
[A"] = 0.004 00 + [H+]
K - 1 75 x 10-5 - rH+K0.00400 + [H+l)
_
+
[H ]
10
K&- 1.75xl0> .00200-[H
]
=*
"
*b)
0
=> pH = 5.06
[HA|
0.001 99 M
[A"] = 0.004 01 M
6
M
If you used Ka = lO -4 "* instead of rounding to 1.75 x 10-5, then [H+] = 8.71 x 10"6 M.
(b) We use Goal Seek to vary cell B5 until cell D4 is equal to Ka.
A
1
2
3
4
5
6
7
8
9
8-43.
A
Ka = 10 -pKa =
Kw =
FHA =
FA =
H =
OH = Kw/H =
pH = -logH [HA] =
[A-l =
B
|c |
n
E
1 75E-05
Reaction quotient
1.00E-14
forKa =
0.002000
;[H+][A-J/[HA] =
0.004000
1.75E-05
<-Goal
Seek
solution
8.693E-06
D4 = H*(FA+H-OH)/(FHA-H+OH)
1.15E-09
5.Ö6Ö8262
0.0019913
B8 = FHA-H+OH
0.0040087
B9 = FA+H-OH
(a) If wc dissolve B and BI l + Br (where Br is an inert anion), the mass balance is
FßH+ + FB = [BH f ] + [B] and the charge balance is [Br] + [OH-] = [BH+] +
[H+]. Noting that [Br] = FQH+, the charge balance can be rewritten as
[ B H + ] = FBH+ + [ O H - ] - [ H + ]
(A)
Substituting this expression into the mass balance gives
[B] = F
B
- [ O H ] + [H+]
(B)
If we assume that [B] = 0.0100 M and [BH+] = 0.0200 M, we calculate
pH = pKa + log T ^ J - 12.00 + log l ö ^ j j = 11.70
If we do not assume that [B] = 0.0100 M and [BH+] = 0.0200 M, we use
Equations A and B. Since the solution is basic, wc neglect [H4] relative to
[OH] and write [B] = 0.0100 -JC and [BH+] = 0,020 0 + x, where x =
[OH"].
so
Chapter 8
Then
we can sav K
10-200
tnenwecansay
Ahb = 10w --
í B H + l í Q H ' l -- ( 0 Q 2 i00° 0+- *)
. r )(*)
[BJ
{00
pH = - l o g - ^ = 11.48.
JC = 0.003 0 3 M
(b) We use Goal Seek to vary cell B5 until cell D4 is equal to Kb.
1
2
3
4
5
G
7
8
9
A
Kb =
Kw =
FBH =
FB =
OH =
H = Kw/OH =
pH = 4ogH =
BH =
B=
B
|C
D
E
1.00E-O2
Reaction quotient
1.00E-14
for Kb =
0.02
[OH-)[BH+J/[BJ =
0,01
0.01|
3.028E-03|<-Goal Seek solution
3.303E-12!
D4 = OH*(FBH-H+OH)/(FB+H -OH)
11.481121
0.0230278
C8=FBH-H+OH
0.0069722
C9 = FB+H-OH
[HPOä-][H+]r Hpo a- rH>
8-44.
Ka =
rTTr^i
= 10- 7 2 0
t H 2 P °4]r H2 P0 4 -
To find pH, rearrange the K& expression to solve for the activity of H + , which is
[H + ]YH+:
***
-
[HP05], H p o ,
At p = 0.1 M, the activity coefficients are y,.+ = 0.83, / „ _„_ • 0.775, and
**
H2PO4
^HP 2- = 0.355, so
4
10 -7.20[H 2 PO 4 -K0.775)
[H+]YH+
=
[HPO2-](0.355)
"
138
*
10 7 W h c n [ H 2 P
"
pH = -logJ4 H + = -log[H+]vH+ - -log(l .38 x 10-7) =
8-45.
Equilibria:
[A1QH2+1ÍH+1
[A13+]
[Al(OH)¿][H+]2
ß2 =
[A13+]
[Al(OH)3(flg)][H+]3
ß3 =
[A13+]
[Al(OH)4][H+]4
ß4[A13+]
[Al2(OH)V][H+]2
K22 =
[Al3+]2
ßi
° 4 l * [Hp024"l
686
(»)
(b)
(c)
(d)
(c)
81
Monoprotic Acid-Base Equilibria
[Ab(OH)54+][H+]4
^43 [Al 3+ ] 3
^
Ky, = [H+][OH-]
(g)
Charge balance: 3[A13+] + 2[A10H2+] + [Al(OH)2 ] + 4[A12(0H)42+ ] +
5[A13(0H)54+ ] +[H+] = [Al(OH)4 ] + [OH-] + [C104 ] (h)
Mass balances: 3F = [CIO;]
(¡)
F = [A13+] + [A10H2+] + [AI(OH)| ] + 2[A12(0H)42+ ] +
[Al(OH) 3 (^)] + [AI(OH)4] + 3[Al3(OH)54+]
(j)
Wc have 10 equations and 10 unknowns, so the problem can, in principle, be
solved.
CHAPTER 9
POLYPROTIC ACID-BASE EQUILIBRIA
The A"a reaction, with a much greater equilibrium constant than A"b, releases H + :
HA" ^ H+ + A2
Ka
Each mole of H *" reacts with one mole of OH" from the Kb reaction:
HA" + H 2 0 ^ H2A + OH".
The net result is that the Kb reaction is driven almost as far toward completion as
the Ka reaction.
R
+ Ï
H3N " ^ C 0 2
(XT3-C02-+
N
H
P^ values apply to -NH3, -C0 2 H, and, in some cases, R.
H,0
N
( + > - C 0 2 - + OH"
=4.37 x 10 4
TÇ
^2
v2
0.100 x
= Kl
F=
C + ^-CCsH + OHN
H2
=>x = 3Mx
10-3 =
[H +]
/Vb2= ^ = 8 . 9 3 x 10-13
'
= [HA"] => pH = 2.51
[A 2 ] = Kl1^
[H2A] = 0.100-Jc = 0.0969 M
(b)
Kb] =
H2
C+>-C02-+H,0
N
H2
(a)
=i
]
= LOO x 10-8 M
¡K]K2F + KiKw
[H+] ~ -W
-^Tf
= 1.00 x 1(^6 => pH = 6,00
[HA"] « 0.100 M
[H2A] = ^ à 2 =
2
,.00 x 10-3 M
=> x -
[OH"] = [HA"] = 3.16 x 10-4 M => pH =
[A2-] = 0.100 -JC =9.97x 10-2 M
0.100MNa 2 A
S g ö l . ,.00 x ,0-3 M
K
(c) O J O O , ^ = ^
0.100 M H2A
O.lOOMNaHA
[A 2-] =
pH
2.51
6.00
10.50
[H2A] = t H + H H A 1 =
[Hx2A]
9.69
10-2
1.00 x 10-3
1.00 x 10-10
82
1 0 0 x
[HA"]
3.11x10-3
1.00x10-'
3.16x10-4
10.50
10-10M
[A2-]
1.00x10-8
1.00x10-3
9.97x10-2
Polyprotic Acid-Base Equilibria
9-5.
83
(a) H2M = H + + HM"
F- x
x
v
0
K\ = 1.42 x LO*3
|QQ„ V = *i => v • 1.12 x 10-2 => pH = -log* = 1.95
[H2M] = 0.100 -x = 0.089 M
[HM"] = * = 1.12 x 10-2 M [M2-] =
/iV|/r2(0.ioo) + /Ci/:w
(b)
[ip] = ^j
[M2] =
KZ2[HM"]
l
J
H+
= 2.01x10-6 M
.
= 5 3 0 x l0 5
%+oioo
[HM"] ~ 0.100 M
[UM-]K
2
J
~ =» P
„
^
H =
428
0
[H2M] = f H I V Hi H 1 = 3.7 x 10 3 M
= 3.8x10-3 M
The method of Box 9-2 would give more accurate answers, since [HM"] is
not that much greater than [I l2M] or [M2-] in this case.
(c)
M2- + H 2 0 ^ HM" + OH"
Kb\ = Kw/Ka2 = 4.98 x 10 9
F- x
x
x
2
K
5
,
v
t
A
,
v
K
,
V
6
0.100-JC =
" ATbl
" -=>
^ * = ^2.23 x lO- ^=> pH
" = - l o gx- f = 9.35
[M 2 ] - 0.100-A- - 0.100 M
[HM"] = x = 2.23 x 10'5M
[H 2 M]=™°=^ X ^ 2 M
9
"6.
IIN^NH
+ H20 -
H N ^ N H 2 + OH" ^ b | = ^
F- x
A" 2
c
x
= Kb\ ^
JC - 3.99 x 10-3
x
=> p H = -log ATW/JC = 11.60
M
[BH+] = x = 3.99 x 10"3 M
[B] = 0.300 -JC = 0.296 M
ÍBH^H!]„215¡<|0.,M
[BH2t] =
9-7.
= 5 . 3 8 x 10 5
For H2A, K\ = 5.62 x I 0 2 and K2 = 5.42 x 10 5
First approximation ([HA"]| « 0.001 00 M):
//riK2(o.ooioo) + Kitfw
,
„
Kt+ 0.001 00
= 2 ' 3 1 * , ( H M => P H '
[H+]l = \j
[H+],[HA"Ji
[H2A]i = ^
^
"• = 4.10 x 10-6
M
=
3 M
84
Chapter 9
K2 [IIA"]|
L
[A 2 '], =
= 2.35x10^ M
t ir]
Second approximation:
[HA"]2 = 0 . 0 0 1 0 Q - [ H 2 A ] I - [ A 2 - ] | = 0.000761 M
¡K¡K2lfiM076\)TK¡K^,
[H + ] 2 = - y
" = 2.02 x 10-4 M => pH2 = 3.70
Kl+
0000761
[H + ] 2 [HA"]2
[H 2 A] 2 = ^ ^
" = 2.73 x 10-6 M
-,
K2 [HA"]2
2
[A "]2 =
[H+ ] 2
=
204><I0
"4M
Third approximation:
[HA-]3 « 0.001 00 - [ H 2 A ] 2 - [ A 2 - ] 2 = 0.0OO793 M
[ H i s - -\j
/Ki*2(0.000793) + /riXw
JC,+0.000 793
"
2 06 x
'O"4 M -> pH3 = 3.69
[H+]3 [HA"]3
[H2A]3 = ^ ^
= 2.90 x 10-6 M
,
1 1 3
9-8.
(a)
=
^2 [HA"]3
[H + ] 3
"
Charge balance:
[K+] + [H+] = [OH"] + [HP"] + 2[P2-]
[K+] = [H2P] + [HP"] + [P -]
....
EqUlllbna:
"4M
2
Mass balance:
R
2 0 9 X 10
(2)
[H+]YHMHP"]yHp-
„
K{
(1)
=
K2
[H 2 P]y H2P
«
[H']YH+[P2-]Yp2-
(4)
[HP"] YHP-
*w = [H + ]y H MOH"]y 0|1 -
(5)
Solving for [K4] in Eqns. (1) and (2) and equating the results gives
[H2P] + [H+] - [P2] - [OH"] - 0
Making substitutions from Eqns. (3), (4), and (5), wc can write
[H+]yH+ [HF] YHpK
l YH2P
* 2 [ H P - ] YHPL
which can be rearranged to
J
+
*W
" [H ] Y„+ Yp2- * [H+] y H+ y 0 H - " °
85
Polyprotic Acid-Base Equilibria
/Cl*2lHP-]Y„p-Y„2p
K]KwyHP
+•
Y„+YP2-
[H + ]
(b)
+
YH^OH"
+
^irH 2 P [HP-]Y H Y|ip-
The ionic strength of 0.050 M KHP is 0.050 M, since the only major ions
are K+ and HP".
[HP"]~ 0.050 M, y HP - = 0.835, yp2- - 0.485, yH2p a 1.00,
yH+ = 0.86, y OH - = 0.81. Using these values in the previous equation
gives [H+] = 1.09 x 10-4 => pH = -log[H + jy H + = 4.03.
9-9.
Case (a): pH = 6.002, [HM-] = 9.80 x 10-3 M , [H2M] - 9.76 x 10-5 M,
[M2-] = 9.85 x 10-5 M
Case (b):
A
I
B
I
C
I
D
|
E
F
G
H
I 1
1 Box 9-1 Successive Approximations
2
4
1st approx. 2nd approx. 3rd approx. 4th approx. 5th approx.
3 pK„,=
5(HA1=
0.01000
0.003675
0.007675
0.005146
0.006745
4 pKa 2 =
J
15th approx.
0.00613201
0.0001 [H*]=
3.15E-05
3.12E-05
3.14E-05
3.13E-05
3.14E-05
3.14E-05
0.00001 (H2AJ =
3.15E-03
1.15E-03
2 41E-03
1.61E-03
2.12E-03
1.92E^3
3.18E-03
1.18E-03
2.44E-03
7 F =
0.01 [A*1 =
4.50
4.51
4.50
1.00E-14 pH =
8 K.«
9
10 Coll D4: [HA] = F
11 Cell D5: [H '] =SQRT((Ka1 *Ka2*04+Ka1 -Kw)í(Ka1 +D4))
1.64E-03
2.15E-03
4.50
4.50
1.95E-03
4.50
5 K.,=
6
«*,=
12 Cell D6: [HíAl = D4*D5/Ka1
2
13 CellD7: ]/K ] = Ka2*D4/D5
14 Cell D8: pH=-log10{D5)
15 Cell E4: ÍHA1 = F-D6-D7
1G After oompuling E4, then highlight cells D 5:E8andFIL . RIGHT
17 After completing column E, highlight cells E4:F:8 and F ILL RIGHT
18 Continue to highlight each column and FILL RIGHT
9-10.
Mass balance: F = [Na+] = [H2A] + [HA-] + [A 2 ]
Charge balance: [Na+] + [H+] = [HA ] + 2[A2'] + [OH ]
Equilibria:
[H2A] = [H+][HA]/K,
[A 2 ] = K2[HA }i[\C]
[OH] = KJ[H+]
Substitute [Na+] = [H2A] + [HA] + [A 2 ] from Equation A for [ N V ] in
Equation B:
[H2A] +JHA-T+ [A>f + [H+] = 4 H * T + tfA2'] + [OH]
[H2A] +[H + ] = [A 2 ] + [OH-]
(A)
(B)
(C)
(D)
(10
(F)
86
Chapter 9
Substitute expressions for [H2A] from Equation C, [A 2 ] from Equation D, and
[OH ] from liquation (E) into Equation F:
11 l+][HA-]/K, + [H+] = K2[UA-)/[H+] + KJ[ti+]
Multiply all terms by [H*] and factor [H f ] 2 out on the left side of the equation:
[H+]2[HA-]/tf, + [ l t ] 2 = K2[HA] + KW
[H+]2{[HA-]/*i +1} = K2[HA'] + KW
Solve for [H+]:
IK2\HA1 + KV _
jK^HAl
+ _
L J
" " A/ [HA]//:, + 1
A/
+
[HA] + K,
K^
The last expression is equivalent to Equation 9-10. Substituting [HA"] « F gives
Equation 9-11.
9-11.
["H 2C03"] = [CO2 (aq)] = KPC02 - 10" 5. 10-34 = H H - 9 M
H 2 C0 3 Ç* HCO3 + H +
/i a i = 4.46 x 10-7
9
10-4- -x
x
x
2
x
10-4 9 - ^ = *•! =* Jf = 2.l6 x 10-«M => pH = 5.67
9-12.
,
*a2 [HCO3]
(a) From Equation C, [CO 3] =
fH+
c
c
o r,!^^-!
(F)
^ a i [CQ2(qg)]
From Equation B, [HCO3] =
(G)
rH+i
Substituting [HCO3] from Equation G into Equation F gives
[CO 3] -
(H)
[H+]2
Substituting for [C02(a<7)] from Equation A into Equation H gives
2
[CO3] =
^a2^al ^H ^C0 2
ffPJ2
(I)
(b) For PC02 = 800 pbar, pH = 7.8,0°C, we find
->
^a2 ^al K\\
PQ02
[°°31 = — f i F F ^ =
IQ-9.3762 m o l
1Q-6.I004 m o l k g -l 10-1,2Q73 m o |
[10-7-8 mol kg" I ]2
5
= 6.6 x 10- molkg'
For l\:o2 - 800 pbar, pi I = 7.8, 30°C, we find
C
2
[ °3l -
kR -l
^32^1
K\IPçQ2
¡yfp
=
kfi -l b a f -l ( 8 0 0 x 1Q-6 b a r )
Polyprotic Acid-Base Equilibria
87
10-8.8324 mol kg-' 10-5-8008 mol kg-' 10-16048 m o | kg-l bar' (800 x 1Q-6 bar)
[lO-^molkg" 1 ] 2
= 1.8 x lO-4 mol kg-'
(c) The equilibrium expressions for aragonite and calcite are
CaC03(.v, aragonite) ç* Ca 2+ + C023"
Kf* = [Ca2+][CO 3 ]
(D)
= 10-6 " 13 mol2 kg-2 at 0°C
= 10-6.1391 m o r2 k g -2 a t 30°C
CaC03(jy, calcite) ^
Ca 2+ + C023"
Kfpl = [Ca2+][C023"]
(E)
= 10-6-3652 mol2 k g 2 at 0°C
= 10-63713 mol2 kg"2 at 30°C
The reaction quotient at 0°C is
[Ca2+][C023"] - [0.010 mol kg'][6.6 x 10"5 mol kg-']
= 6.6 x 10-7 mol2 kg"2 = lO"6-'* mol2 k g 2 < 10f> " 1 3 mol2 kg-2,
so aragonite will dissolve.
But lO"6-18 mol2 kg"2 > 10-6-3652 m o i2 k g-2 j s o calcite will not
dissolve.
The reaction quotient at 30°C is
[Ca2+][C02~] = [0.010 mol kg-'][1.84 x 10-4 mol kg-1]
= 1.84 x 10-6 mol2 k g 2 - 10-5-74 mol2 kg"2 > 10-6" 13 m ol 2 kg"2,
so neither aragonite nor calcite dissolve.
[CO 2 ]
9-13.
pH = pKa + log
9-14.
We begin with (25.0 mL)(0.023 3 M) = 0.5825 mmol salicylic acid (H2A,
[HCO3]
( x g)/( 105.99 g/mol)
„ rtA
10.00 = 10.329 + log ¿.OQ g)/(84.01^mol) = > * = 2.96g
pK\ = 2.972, pAT2 = 13.7). At pH 3.50, there will be a mixture of H2A and HA".
H2A +
Initial mmol:
0.5825
Final m m o l : 0 . 5 8 2 s - J C
OH"
JC
3.50 = 2.972 + log o,582 5 -;r ^
->
HA"
—
X
*=
0449
3
(O.4493 mmol)/(0.202 M) = 2.223 mL NaOH
mmo1
+
H20
88
9-15.
Chapter 9
Picolinic acid is HA, the intermediate form of a diprotic system with pA'i = 1.01
and ptf2 = 5.39. To achieve pH 5.50, wc need a mixture of HA + A".
Initial mmol:
Final m m o l :
IIA
+
10.0
I().0-jc
5.50 = 5 . 3 9 + l o g j Q Q ^
OH"
JC
—
-»
A—
x
=> A - = 5 . 6 3 m m o l ~ 5.63 m L N a O H
Procedure: Dissolve 10.0 mmol (1.23 g) picolinic acid in » 75 mL H 2 0 in a
beaker. Add NaOH (=: 5.63 mL) until the measured pH is 5.50. Transfer to a 100
mL volumetric flask and use small portions of 1120 to rinse the contents of the
beaker into the flask. Dilute to 100.0 mL and mix well.
9-16.
At pH 2.80, we have a mixture of SO;]" and HSO4 , since pKa for HSO4 is 1.99.
[SO2"]
2.80 = 1.987 + l o g j j j ^ => HSO4 = 0.153g [SO 2 ]
The reaction between H 2 S0 4 and SO2" produces 2 moles of HSO4 :
Initial mmol:
Final mmol:
H 2 S0 4 + SOj- -* 2HSO4
x
y
—
—
y-x
2x
The Henderson-Hasselbalch equation told us that [HSO4 ] = 0.153« [SO2,"]
=> 2x = 0.153g (y-x).
Since the total sulfur is 0.200 M,,v + ^ = 0.200 mol.
Substituting JC = 0.200 - y into the equation 2x - 0.153s 0 - -v) gives
Na 2 S0 4 =y = 0.1867 mol = 26.52 g and H 2 S0 4 = x = 0.013 3 mol = 1.31 g.
9-17.
pK2 for phosphoric acid is 7.20, so it has a high buffer capacity at pH 7.45 (from
the buffer pair H 2 P04 /HPOj"). At pH 8.5, the buffer capacity of phosphate
would be low and it would not be very useful.
9-18. NH3
NH3
K
NH3
K
NH2
K
CHCH2CH2C02H== CHCH2CH2C02H == CHCH2CH2C02- == CHCH 2 CH 2 C0 2
C0 2 H
C0 2 glutamic acid
COf
COf
89
Polyprotic Acid-Rase Equilibria
NH3
NH3
CHCH^Q^OH - CHCH2-^0)-OH 5
C02H
C0 2
tyrosine
NH2
9-19.
NH2
ft
CHCH 2 -<Q>-OH ^
ÇHCHr^^"0'
C02"
C02"
/A'iK2(0.05OO) + KiKw
(a) For0.0500 M KH2P04, [H'] = -\J
^ + 0.0500
=
]
"
10
*
=> pH - 4.70
4.70 = 2.148
+
log
[H2PO4]
¡ïSJï^J
[H3P04j
=> ^ ^
= 2.8x10-3
/A-2A3(O.O5OQ)4-A:2A:W
(b)
For0.0500MK2HPO4,[H + ]=M
K 2 +0.0500
= 1
-"x
lrt
l0 10
"
=> pH - 9.70
[H2PO4]
[H3P04]
9.70 = 2 . 1 4 8 . log Ï Ï Ï A ] ^ Ï Ï Ï ^ = 2.8x,0-«
pATl =2.148
9-20.
(a)
H 3 P0 4
—
pK2 = 7.198
H2PÛ4
-^
HPO2,'
pK 3 = 12.375
î
Î
pH = (2.148+7.l98Y2
pH =(7.I98+I2.375V2
4.67
—
PO^
- 9.79
pH 7.45 corresponds to a mixture of NaH2P04 and Na2HP04. (You could
get the same result by mixing other combinations such as H3P04 and
Na 3 P0 4 or H 3 P0 4 and Na2HP04.)
[HPO3-]
(b) pH = pK2 + log [H P0 ]
2
4
[HPO2]
7 ^ = 7.l98
+
[HPO2-]
tog555S=>55^-L7^
Combining this last result with [HPOj" ] + [H2P04 ] = 0.0500 M gives
[HPOj- ] = 0.032 O5 M and [H2P04 ] = 0.017 9 5 M. Use 4.55 g of Na2HP04
and2.15gofNaH 2 P0 4 .
90
Chapter 9
(c) Here is one of several ways: Weigh out 0.050 0 mol Na 2 HP0 4 and dissolve
it in 900 mL of water. Add HCl while monitoring the pH with a pH
electrode. When the pH is 7.45, stop adding HCl and dilute up to exactly
1 L with H 2 0.
9-21.
Lysine hydrochloride (H2L+) is
NH
I
CHCH2CH2CH2NH3
co2
*
,.,
,
/à'|K 2 (O.OIOO)+A:|/£: W
lor which [ I l |
AÍ
K]+0.0100
a 232
x 10
M
=> P H = 5.64
[H2L+] = 0.0100 M
[H 3 L 2+ ] = - H + ] ^ " 2 L + ] = 1.36x10-6 M
A: 2 [H 2 L+]
[HL]
9 22
+
- - 7"X/
^ p
CC
^
1
H
A^IIII I
= 2.40 x io-ll M
+
p^-1.6 HN-^
NH 3
II
[L-] = -j^r1
= 3.68 x 10-6 M
CO
»
.2-
HjHiS^
NH 3
UnUis'
HistuiíiK-
pK3 = 9.28
-fc
L
H
H
NH2
His
Histidine hydrochloride (FM 191.62) is H2His+Cl-, the intermediate form
between pK\ and pK2. A pH of 9.30 requires neutralizing all of the H2His+ to
HHis and then adding more KOH to create a mixture of and HHis + His\
Therefore, we must add 1 mol KOH for each mol of His-HCI to get to HHis and
then add some more KOH to obtain the mixture of HHis and His". Initial mol of
His-HCI = 10.0 g/(191.62 g/mol) = 0.052 1 9 mol. We require 0.052 1 9 mol of
KOH plus the amount JC in the following table to obtain the correct mixture:
Initialmol:
Final mol:
HHis
0.0521 9
0.052
Iç-JC
+
OHx
-•
His—
JC
91
Polyprotic Acid-Base equilibria
PH
= pK3 + l o g ^ H j
=> 9.30 = 9.28 + log Q 0
S 2
\
x
=> v = 0.026 7o
Total mol KOH required = 0.052 19 + 0.026 7 0 - 0.078 9 mol
= 78.9 mL of 1.00 M KOH
9-23.
(a)
[C3-] 7C3pH = pK2 (citric acid) + log ^ H c 2 . j y
~
(1.00X0.405)
PH = 6.396 + log ¡2.00X0.665) =
5M
(b) If the ionic strength is raised to 0.10 M,
PH
9-24.
(a)
= 6.396 + log {1™WQ3I)
= 5-59
(b) A-
HA
(c) pH = pKa + jjñfi
7.00 = 7.00+ log^Ty^
[A"]/[HA] = 1.0
6.00 = 7.00 + logrpiXj
[A']/[HA] - 0.10
9-25.
(a)
4.00
(b) 8.00
(c) H2A
9-26.
(a)
9.00
(b) 9.00
(c) BH1
(d) 12.00 - 9.00 + log
9-27.
O
¿
ÍB1
[BH+]
°^>/li
O—P-O—v. ^ L ^O
- ut
+
N
H
(d) HA"
(e) A2"
[B]/[BH+] = 1.0 x 103
92
9-28.
Chapter 9
Fraction in form HA = a H A
10"5
= 1 0 - 5 - n ( H = 0091.
111'I
= [H+] ^
Ka
Fraction in form A" = a\- = r „ + 1 „ = 0.909.
[H J + Ka
fA'1
[HA]
9 29
" -
a
H2A =
aAôîî7~
=
[H+]2
=
"*'wmcn
ma
k e s sense.
[H+l 2
(VV]lKl+KlKÏ where [H+] = 1 0 - 7 0 0 , K l =
+
10-8.00,and
K2 = 1 0 - 1 0 0 0 ^ a H 2 A = 0.91
9-30.
9-31.
aH2A
8.00
0.877
«HAa A 2-
0.123
4.54 x 10-4
pHj
aH2A
LOO
0.893
«HAa A 29-32.
10.00
0.049 6
PH
PH
0.694
0.257
L92
0.500
0.107
0.500
5.76x10-7 2.23x10-5
6JH)
6.27
5.41 x 10-5 2.23 x 1 0 s
0.651
0.349
10.00
1.55 x 1 0 1 2
0.500
0.500
1.86 x l(H
0.999 8
(a) The derivation follows the outline of Equations 9-19 through 9-21. The
results are
[H3A]
[H+]3
«H3A 2
+
[u+]KlK2 + K\K2K3
F
[ H + ] 3 + [U+] Kl
[H + ] 2 /T|
[H • ]3 + [H + ]2tf| + [H+\K\ K2 +
[H 2 A~]
ttH2A
F
"
aHA2.
HA
„A,
A
=
.
IHA^I
F
IA±1
F
=
KxK2Ki
[H+] KXK2
[H ]3 + [H ] tf, + [H+]K,K 2 + K\K2K3
+
+ 2
*1* 2 *3
[H + ]3 + [H+p/Ci + [H+]KiK2 +
K\K2Ki
(b) For phosphoric acid, pK\ = 2.148, pK2 « 7.198, and pK3 = 12.375. At
pH = 7.00, the previous expressions give aH 3 A = 8.6 x 10-6, CIH 2 A- •
0.61, a H A 2 - - 0.39, a n d a A 3 - = 1.6 x 10A
« , PH - *.w*{g¡¡ • — » , 5 + log g • g - 0,6,
fraction unprotonated =
[NH 3 ]
O.569
7- = 77-77—rr = 0 36
[NH 3 ] + [NHÍ]
°-569 + 1
93
Polyprotic Acid-Base Equilibria
9-34.
The quantity of morphine in the solution is negligible compared to the quantity of
cacodylic acid. The pH is determined by the reaction of cacodylic acid (HA)
with NaOH:
HA
1.000
0.200
Initial mmol:
Final mmol:
+
OH"
0.800
-•
A"
+
H20
0.800
0.800
pH = pKa + log JAJ. = 6.19 +log
- 6.79
[HA]
" • " *"*» 0.200
For morphine (B), Ka = Kw/Kb = 1.0 x 10"14/1.6 x 10-6 = 6.25 x 10-9
=> pKa - 8.20
At pH 6.79, we can write pH = p#BH+ + l°g
m
6.79 - 8.20 +log T T ^ T
BH
Fraction in form BH
9-35.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
9-36.
f
9
[BH+]
fBH+1
[B] + [BH+]
= 0.039 => [B] - 0.039 [BH+]
fBH+1
"¡T = 96%
0.039 [BH+] + [ B H ]
A
B
C
Fractional composition for d iprotic acid
K1 =
PH
9.55E-04
3.31 E-05
pK1 =
3.02
pK2 =
4.48
A4 = 10A-A8
A6 = 10A-A10
C4 = 10A-B4
[H+j
1
0.1
2
0.01
_3
0.001
4
0.0001
5 0.00001
6 0.000001
1E-07
7
K2 =
[BH+]
D
E
F
a(H2A)
a(HA")
9.91E-01
0.00946
0.912562 0.087149
0.503369 0.480713
0.072928 0.696455
0.002423 0.231386
3.07E-05 0.029313
3.15E-07 0.003011
«(A2')
3.13E-06
0.000289
0.015918
0.230618
0.766191
0.970656
0.996989
I
D4 = $C4A2/($C4A2+$C4*$A$4+$A$4*$A$6)
A
E4 = $C4*S>A$4/($C4 2+$C4*$A$4+$A$4'$A 56)
F4 = $A$4* $A$6/($C4 *2+$C4*$A!&4+$A$4*$/^$6)
1.0
H2A0.8
H
0.0
Ai • /
^
/HA "
\v »
\ +
a.
< 0.4
0.2
/A*
2
f\
/ *
// •
v V
1
/
7
PH
/
\
10
\
11
12
13
94
Chapter 9
A
B
C
E
F
G
D
1 Fractional composition for triprotlc acid
2
a(H3A)
u(H2A")
a(HA2")
a(A3")
3 K1 =
[H+l
PH
4
3.74E-02 8.01 E-10 7.82E-20
3.89E-03
9.63E-01
1 1.00E-01
5 K2 =
1.00E-02
7.20E-01
2
2.80E-01 5.99E-08
5.85E-17
2.14E-09
1.70E-06
1.66E-14
1.00E-03
2.04E-01
6
7.96E-01
3
1.00E-04
4
7 K3 =
2.51 E-02 9.75E-01 2.08E-05 2.04E-12
8
9.77E-12
5
1.00E-05 2.56E-03 9.97E-01 2.13E-04, 2.08E-10
1.00E-06
9 pK1 =
2.56E-04
6
9.98E-01 2.13E-03 2.08E-06
1.00E-07 2.52E-05
10
2.41
7
9.79E-01 2.09E-02
2.05E-06
11 p K 2 1.00E-08
8
2.12E-06 8.24E-01 1.76E-01I 1.72E-04
12
8.14E-08
8.67
1.00E-09
9
3.17E-01 6.77E-01 6.61 E-03
13 pK3 =
10
4.09E-02 8.74E-01
1.05E-09
8.54E-02
1.00E-10
14
11.01
11
2.36E-03 5.05E-01 4.93E-01
1.00E-11 6.07E-12
15
12
4.34E-05 9.28E-02
1.00E-12
1.12E-14
9.07E-01
16
13
1.00E-13 1.22E-17 4.74E-07
1.01 E-02 9.90E-01
17 A4 = 1 0 * ^ 0
A
18 C4 = 10 -B4
19 D4 = $C4A3/($C4A3+$C4A2*$A$4+$C4*$A$4*$A$6+$A$4*$j\$6'$A$8)
20 E4 = $C4A2*$A$4/($C4A3+SC4A2*$A$4+$C4*$A$4*$A$6+$/ \$4*$A$6*$yK$8)
21 F4 = $C4*$A$4*$A$6/($C4A3+$C4A2*$A$4+$C4*$A$4'r$A$6+$A$4»$A$6*$A$8)
22 G4 = SAS4'SAS6*$A$8/($C4A3+$C4A2*$A$4+$C4*$A$4*$A$6+$A$4*$A$6*$A$8)
95
Polyprotie Acid-Base Equilibria
9-37.
(a) Fractional composition in tetraprotic system
A
It
C
D
E
F
G
H
I
Fractional composition in tetraprotic system
1
2
3
4
5
6
7
8
9
10
11
12
13
14
IS
16
17
18
19
20
21
22
23
Kal =
pH IH+1
1E-0I
1.58F.-04
1
1E-02
2
Ka2
1 F.-03
3.98E-07
3
1R-04
Ka34
1 E-05
3.98E-07
5
11-Od
6
Ka4 =
1E-07
3.98E-12
7
1E-08
8
IE-09
9
IE-10
10
IE-11
11
1E-12
12
IE-13
13
Denom, Alph(H4A) Alph(H3A) Alph(ll2A) Alph(IIA) Alph(A)
2.5E-14 9.9E-25
6.3E-09
1 OF. - 00 1 6E-03
I.OE-04
9.8E-2I
6.2E-07
1.6 E-02
2.5E-11
9.8E-01
1.0E-08
2 2\ -iiK
1.4E-01
8.6E-17
5.4E-05
1.2E-12
8.6E-01
2.4E-03 9.7E-06 3.9E-13
2.6E-I6
3.9E-0I
6.1E-01
3.6E-02
I.4E-03
5.7E-10
1.7E-I9
5.7E-02
9.1E-01
4.0E-07
2.5E-01
I.0E-01
2.5E-22
4.IE-03
6.4E-01
7.6E-01
3.0E-05
3.3E-24
3.0E-05
4.8E-02
1.9E-01
9.7E-OI
3.9E-04
6.2E-04
2.4E-02
3.9E-08
2.6E-25
9.9F.-0I
4.0E-03
6.3E-06
4.0E-11
2.5E-03
2.5E-26
3.8F.-02
2.4E-04 9.6E-01
3.8E-14
6.1E-08
2.6E-27
2.XF.-01
4.5E-I0
I.8E-05 7.2E-01
2.9E-I7
3.5E-28
8.0E-01
1.3E-12
5.0E-07
2.0E-0I
I.2E-28
8.0E-21
6.2E-09
2.5E-02
9.8E-01
1.0E-28
9.8E-25
1.5E-15
C4= I0A-B4
D4 = C4A4+$A$4*C4A3+$A$4*$AS6*C4A2+$A$4*$A$6*$AS8*C4
+$AS4*$A$6*$A$8*SA!(10
E4 = C4M/D4
H4 = $AS4*SA$6*$AS8*C4/D4
F4 = $A$4*C4A3/D4
A
14
= $A$4*$A$6*$A$8*SA$I0/D4
G4 = SA$4»SAS6*C4 2/D4
Cr(OH) 3 (aq)
^Cr(OH);
<b)
A > lO"6 84 = [Cr(OH)3(ia7)], so [Cx(OUh(aq)} = 10-684 M = i.4 5 x 10-7 M
M
W
K
a3
_ 10 c 10 _ [CKOH)3(flg)3[H+] rio-^ïïio^QO]
[Cr(OH)î]
[Cr(OH)J]
rio-^ino- 400 !
rr ,™+ T
M 44
=} [Cr(OH)2 ] =
^47j
= 10-4.44 M
96
Chapter 9
32
[Cr(OH)2+]
[Cr(OH)2+]
^ICKon^^'^ir^-io-^M
9-38.
The isoelectric pH is the pH at which the protein has no net charge, even though
it has many positive and negative sites. The isoionic pH is the pH of a solution
containing only protein, H+, and OH".
9-39.
The average charge is zero. There is no pH at which aH molecules have zero
charge.
9-40.
. . . ru+.
A /AWO.OlOj + A ^
Isoionic [H ] = yj
=> pH = 5.72
Kl+{omo)
pA'i + pK2
Isoelectric pH =
~
= 5.59
9-41.
A mixture of proteins is exposed to a strong electric field in a medium with a pH
gradient. Positively charged molecules move toward the negative pole and
negatively charged molecules move toward the positive pole. Each protein
migrates until it reaches the point where the pH is the same as its isoelectric pH.
At this point, the protein has no net charge and no longer moves. Each protein is
therefore focused in one region at its isoelectric pH. If a protein diffuses out of
its isoelectric zone, it becomes charged and migrates back into the zone.
CHAPTER 10
ACID-BASE TITRATIONS
10-1.
The equivalence point occurs when the quantity of titrant is exactly the
stoichiometric amount needed for complete reaction with analyte. The end point
occurs when there is an abrupt change in a physical property, such as pH or
indicator color. Ideally, the end point is chosen to occur at the equivalence point.
10-2.
Ka
pH
0
13.00
1
12.95
9
11.96
5
12.68
9.9
10.96
10
7.00
10.1
3.04
12
1.75
Representative calculations:
„
.
OmL:
. . .
pi I = -log
^w
10-14
-]o% 0.100
=
[OH"]
9
TO
100
13.00
0.089 1 M
pH = 12.95
1 mL:
[Oír] =
10 mL:
[OIF] = [H+] - 10-7 M
10.1 mL:
[H+] = ( T T ^ T ) ( L 0 O ) = 9.08 x 10-4 M => pH = 3 04
(o.i oo)
w
13ÖO
a
Va (mL)
10-3.
Consider the titration curve near the equivalence point. If we titrate strong acid
with strong base, the concentration of H + is close to 1% of its initial value when
we arc 99% of the way to the equivalence point (i.e., when Vb = 0.99 Vc). (This
statement would be exactly true if there were no dilution occurring. We will
neglect dilution.) If the initial acid concentration were, say, 0.1 M, then [H + ] =
1% of 0.1 M = 0.001 M at Vb = 0.99 Ke. The pH is -log(0.001) = 3. At 99.9%
completion, [H + ] = 0.1% of 0.1 M = 0.0001 M and the pH is 4. When the titration
is 0.1% past the equivalence point, [OIL] = 0.0001 M and the pH is
-log(AVOOOOI) = 10. The pH jumps from 4 to 10 in the interval from Vb =
97
Chapter 10
0.999Ke to 1.001 Vc. Even though the concentration of H + hardly changes, its
logarithm changes rapidly around the equivalence point because [H1] decreases by
orders of magnitude with tiny additions of OH- when there is hardly any H+
present.
10-4.
The sketch should look like Figure 10-2. Before base is added, the pH is
determined by the acid dissociation reaction of HA. Between the initial point and
the equivalence point, each mole of OH" converts an equivalent quantity of HA
into A-. The resulting buffer containing HA and A" determines the pH. At the
equivalence point, all HA has been converted to A". The pH is controlled by the
base hydrolysis reaction of A" with H 2 0. After the equivalence point, excess Ol F
is being added to the solution. To a good approximation, the pH is determined
just by the concentration of excess OH\
10-5.
If the analyte is too weak or too dilute, there is very little change in pH at the
equivalence point.
10-6.
Vb
0
1
5
pH
3.00
4.05
5.00
Representative calculations:
OmL:
+
HA = H + A"
0.100 -x
v
9
5.95
9.9
7.00
v2
ÖloÖ^c =
10
8.98
10
=>
10.1
10.96
12
12.25
x = 9 9 5 x l(yA
M
x
=> pH = 3.00
J_mL: pH = pA-a + log 7 ^
I OmL:
A" +
= 5.00+ log £ = 4.05
H 2 0 - HA + OH'
({^(O.lOO)-*
2
K
o 0 9 0 9 - * = ~K
•
=> * = 9.53x 10-6
=> [H+] - -*• => pH - 8.98
10.1 mL:
[OH"]
= ( T ] ^ T ) ( L 0 0 ) = 9.08 x 1(HM => pH = 10.96
99
Acid-Base Titrations
T\
12.5
4
10-7.
[A-]
pH = pKA + log rj , A j
6
8
V b (mL)
10
12
pA-a-l = p / i a + l o g ^ => THA^ = 10
If the ratio r H A is to be TK , then TT of the initial HA must remain as HA.
Atthispoint,[A_]/[HA] = (l/ll)/(10/ll) = l/10.SopH = pA:a-l when Vb =
Ve/U.
In a similar manner, pH = pKa +1 when Vb = lOfyi 1.
For anilinium ion, pKa = 4.601. For the titration of 100 mL of 0.100 M
anilinium ion with 0.100 M OH", the reaction is
<C^NH3
OmL:
+
OH ->
(^~ NH,
+ H-,0 and Fe = 100 mL.
<0)-N.l,
"NU, - c ^ - N H 2 + H+
O.lOO-x
0.100-x
x
= K*=l0~4(>0
x
= » x - 1 . 5 7 x l 0 - 3 ^ p H = 2.80
VJl 1 = 9.09 mL: pH = pKa- 1 = 3.60
VJ2 = 50.0 mL: pH = pKa = 4.60
\0VJU =90.91 mL: pH = pKa+\
= 5.60
+
K.= 100.0 mL: BH has been converted to B.
B + H20 ^
F-*
BH+ + OH"
x x
=>x = 4.46* 10-6 M
K rt
©(0.100)-*
Pi I • - l o g — - 8.65
1.2K.= 120.0 mL: There arc 20.0 mL of excess NaOH.
100
Chapter 10
[OH"] = (^)(0.100) = 9.09 x 10-3 M => p H = 11.96
I I I • I I I • • II) I I I I I I 1 ] -
2
I i i i
0
20
40
60
'.'••••
80 100 120
Vb(mL)
10-8.
The titration reaction is HA + OH" - • A" + H 2 0. A volume of ^mL of HA will
require 2V mL of KOH to reach the equivalence point, because [HA] - 0.100 M
and [KOH] = 0.0500 M. The formal concentration of A" at the equivalence point
will be [ T ^ P J ( 0 . 100) = 0.033 3 M.
_^
A' • H20 «* HA • OH"
0.033 3 - j e
10-9.
Q
x
x
The pH is found by writing
x2
0^333^ = *
, _A
Kw
l 0 * 1014
=t " Ï ^ T P
. w . .
__
_, „_
=> X= 1.50 x lO"6 M -> pH = g.lg
+NH-
+ H+
Ka m 10-627
H+
+
OH" ^ ^
H20
\/Kw = 1014
• ILO
+ OH
K = -p- = 5.4 x lO7
10-10.
HA
+
OH"
+ H20
Initial mmol: 5.857
Final mmol: 5.857-x
pH = 9.24 = pA-a + log j ^ j = 9.39 + log JJ^~^
=>* = 2.4 28 mmol
101
Acid-Base Titrations
[QH"]=
2,428 mmol
22.63 mL " °
107 M
(CH 3 ) 3 NH + +
OH-
10-11.
Initial mmol:
1.00
0.40
Final mmol:
0.60
—
-•
(CH3)3N
+
H20
O40
First, find the ionic strength:
[(CH3)3NH+] = 0.60 mmol/14.0 mL = 0.042 86 M
[Br"] = LOO mmol/14.0 mL = 0.071 43 M
[Na+] = 0.40 mmol/14.0 mL - 0.028 57 M
p = {le??
= 0.071 M
[B]YB
pH = pKa + log [ B H + ] y B H +
„
, (0.028 6)( LOO)
n „ n n
pH = 9.799 + log ¡0.042 9)(0.80) =
nmt.
972
In the previous calculation, we used the size of (CH3)3NH+ (400 pm) and the
activity coefficient interpolated from Table 7-1.
10-12.
The sketch should look like Figure 10-9. Before base is added, the pH is
determined by the base hydrolysis reaction of B with II 2 0. Between the initial
point and the equivalence point, each mole of H + converts an equivalent quantity
of B into BH+. The resulting buffer containing B and BH+ determines the pH. At
the equivalence point, all B has been converted to BH+. The pH is controlled by
the acid dissociation reaction of BH+. After the equivalence point, excess H + is
being added to the solution. To a good approximation, the pH is determined just
by the concentration of excess H + .
10-13.
At the equivalence point, the weak base, B, is converted completely to the
conjugate acid, BH + , which is necessarily acidic.
10-14.
Ka
0
1
5
9
9.9
10
10.1
12
pH
11.00
9.95
9.00
8.05
7.00
5.02
3.04
1.75
Representative calculations:
OmL: B + H 2 O ^ B H + O H "
0.100-jr
x
x
x2
ÔTÔÔ^Jc" 10 "
M
= > * = 9.95 x 10"4 M
£
[H+] = - f => pH = 11.00
102
Chapter 10
lo
JjnL: pH = pKm+ +
r2
B + H+
10 mL: Dil
-too
TTïï/Q-ioo)-*
10-1 mL:
g f ¿ | t ¡ = 9.00 + logy = 9.95
:- 10- 900 M =>x = 9.53* 10-6 M
0.090 9 -x
[H+] =x => pH = 5.02
x
*
[H+] = ( T T O T ) 1 0 0 ) "
9 0 8
* 10"4 M => pH =
3.04
11 O ' i • | > | « i—-—
x
a.
7 -
)
5 -
<)
i
-
. i . i , i . i . i , T
0
2
4
6
8
10
12
V a (mL)
10-15.
The maximum buffer capacity is reached when
V = \Vç, at which time T ¿ ^ T - 1 and pH = pKa (for BH+).
10-16.
^Q)-CH2NH2 +H f ^
{Q)-CH2NH3
¡£¡£5taí
K = l/A:a(forC6H5CH2NH3) = 2.2 x 109
10-17.
Titration reaction: B + H+ —• BH+. Tofindthe equivalence point, we write
(50.0X0.0319) - (Fe)(0.0500) => Kc = 31.9 mL.
A',
OmL: B + H 2 0 ^ BH+ + OH" 0 0 3 1 9 _ J C = *b = Y~ = 2.22 x 10-5
0 0319 - x
12.0 mL:
Initial:
Final:
v
v
B + H+ ->
31.9
12.0
19.9
_
=> x = 8.31 x 10-4M => pH= 10.92
BHH
12.0
[Bl
19.9
pH - pK& + logf^^rp 9.35 + log Y2^ = 9.57
1/2 V,: pH = pKa = 9.35
103
Acid-Base Titrations
1.9
30 mL: pH = pKa + l o g ^ T j = 8.15
V¿
B has been converted to BH+ at a concentration of (^r^j(0.031 9)
= 0.0195 M
x2
BH+ ^ B + H
0~rjÏ95~^ = *a => * = 2.96x 10-6M
0.0195-*
x
x
. M
=^ pH = 5.53
35.0 mL: [H+] = (WV)(0.0500) = 1.82 x 10"3 M => pH = 2.74
+
10-18.
Titration reaction: CN" + H + -+ HCN
At the equivalence point, moles of CN" = moles of 11 '
(0.100 M) (50.00 mL) = (0.438 M)(K e ) => Fc = 11.42mL
(a)
CN"
Initial:
Final:
(b)
+
H+
-•
HCN
11.42
7.22
4.20
_
4.20
7 22
pH = pKa + log ¿ 2 0 = 9.45
1 1.82 mL is 0,40 mL past the equivalence point.
[H+] = ( ^ [ ^ ) (0.438 M) = 2.83 * 10-3 M => p H = 2.55
(c) At the equivalence point, wc have made HCN at a formal concentration of
(6L^)<0JOO)
HCN
0.08I4-*
^
=
00814M
H+
+
x
x
-
CN -
v2
0.0814-.v
=
* a => •*= 7 -l * 1°"6
=> pH = 5.15
104
Chapter 10
r
10-1°.
/*
t
!?2
X
The pli of the initial solution before base
is added is determined by the first acid
dissociation reaction of H2A. As base is
added, it converts H2A into an equivalent
amount of HA". The buffer consisting of
H2A and HA" governs the pH. At the first
Q.
equivalence point, wc have a solution of
"pure" HA", the intermediate form of a
diprotic acid. The pH is determined by the
competitive acid and base reactions of
21
HA". Between the two equivalence points
vb->
there is a mixture of HA" and A 2 \ which is
another buffer. At the second equivalence point, we have converted all HA" into
A 2- , whose base hydrolysis reaction determines the pH. After the second
equivalence point, the excess OIL added from the buret is mainly responsible for
determining the pH, with negligible contribution from A 2 \
10-20.
The protein has an average charge of 0 at the isoelectric point. Since the isoionic
point occurs at a lower pH, the average charge of the protein must be positive at
the isoionic point.
10-21.
The equivalence point could be attained by mixing pure HA plus NaCI.
Neglecting effects of ionic strength, the pH is equivalent to that of a solution of
pure HA. This is the isoionic pH.
10-22.
(a)
HA
B + H+
A" + H*
BH
B + HA r± BH+ + A"
(b)
*a
K = K\JKV
K — K^KyfK-^
= 10-2.86 10-3.36/10-14.00=107.78
In the upper curve, 2VC is halfway between the first and second equivalence
points. The pH is simply pK2> since there is a 1:1 mixture of HA" and A2".
In the lower curve, pK2 (= P&BH+) occurs when there is a 1:1 mixture of B
and BH '. To achieve this condition, all of B is first transformed into BH+
by reaction with HA until Ve is reached. Then, at 2VC one more equivalent of
B has been added, giving a 1:1 mole ratio B:BH+, so pH = pK&n+.
105
Acid-Base Titrations
10-23.
Va
0
1
5
9
10
11
15
19
20
22
pH
11.49
10.95
10.00
9.05
8.00
6.95
6.00
5.05
3.54
1.79
Representative calculations:
OmL:
B + H 2 0 1=' BH+ + OH"
Q.IOO-X
=
">-«»=>*-3.11 * 10-3 M
0.100-Jt
lmL:
pH = - l o g - f = 11.49
lo
pH = pKBH+ +
-[§]__= 10.00+logy = 10.95
g?ÍH+T
10 mL: Predominant form is BH+ with formal concentration Tïô(0.100) = 0.090 9 M
lQ-6.00 10-1000(0,0909)+ lQ-6.00 10-14~0€
[H ] - \j
10-6.00 + 0.0909
+
"V
= 1.00 x 10-8 ^ pH = 8.00
l l m L : pH = pKBH2+ + l o g ^ ^ =
60
BHj 22++ 5=* BH+ + H +
20 mL:
100
j2öt0.100) - x
°
+ lo
g f = 6-95
= 10-6.00 =>x = 2.88x lO-4
0.083 3-Jt
x
pH = 3.54
22 mL: [H+] = (TII)^ ! °°^ =
L64
* , 0 " 2 M =* p H
=
l79
[ I I • I | I I I ^^»»—T-l I | I
X
CL
o ' ••• •*•• • •I ••.. i••- •i
0
5
10
15
20
V a (mL)
10-24.
Vb 0
1
5
9
10
11
15
19
20
22
pH 2.51
3.05
4.00
4.95
6.00
7.05
8.00
8.95
10.46
12.21
Representative calculations:
OmL:
H2A ^
0.100 -x
HA" + H +
x
x
0.100-jc
= 10-4.00 => ^ = 3.11 x 10-3 M
=> pH = 2.51
106
Chapter 10
lmL:
pH = pK\ + logfff^]
= 4 0 0 +
lo
89
=
305
10 mL: Predominant form is HA'with formal concentration
[T77J)(0.100)
0.0909 M.
[H+] «
10-4.00 10-8.00(0.0909)+ 10-4.00 IQ-14.00
! 0-4.00 + 0.090 9
= 9.99x 10-7 -> pH = 6.00
11 mL: pH = pK2 + log-'—- 1 = 8.00 +log ¿ = 7.05
}
[HA"]
i «
20 mL:
A 2 ' + H 2 0 ï ^ HA" + OH"
vx = 2.SSx lO-4 M
0.083 3 -x " TJ
ES
(l2o)(0.100) x
p H = - l o g - f = 10.46
22 mL:
[OH"]
= ( T ^ J O O O ) = L64 x 10"2M => pH =
12.21
2 v. ... i . . . . i .
5
10
15
20
V b (mL)
10-25.
Titration reactions:
HN
NH
+ H+
H2N
NH
+ H+
OmL:
B + H20 ^
0.100-jr
—-
H2N
NH
Vc = 40.0 mL
H2N
NH^
Ve = 80.0 mL
BH+ + OH"
x
x
0.100-x
=
A:W
Kbl - *sr=
a2
•JC = 2 . 2 9 X
10-3 M
1.0 x 10-' 4
1 . 8 6 x 1 0 ' °
= >
p
H
=
11.36
107
Acid-Base Titrations
; j ¿ ^+ •--- 9 . 7 3 1 + l o g y = 1 0 . 2 1
[BH ]
20.0 mL: pH = pK2 = 9.73
30.0 mL: pH = pK2+ log 3 = 9.25
40.0 mL: B has been converted to BH+ at a formal concentration of
F - (|o^o)( 0100 ) =0.0500 M
lK\K2F + K\ky,
[H+] =
K\ + F
•
v
(4.65 x 10-6)(1.86 x 1Q-10)(0.0500) t (4.65 - 1Q-6)(L0 * 1Q-'4)
4.65 x 10-6 + 0.0500
=> pH = 7.53
50.0 mL: pH = pAi + log ¡ g § 5 = 5.333+ logf = 5.81
[BH2 J
60.0 mL: pH = pA'i = 5.33
70.0 mL: pH = pK\ + log} = 4.86
80.0 mL: B has been converted to B H | + at a formal concentration of
Í^^J(O.IOO) - 0.033 3 M
BH+ +
BH
0.0333 -x
90.0 mL:
*
H+
x
0.033 3 - J C
= Ai =4.65 x lO-6
x =3.91 x lO-4 M => pH = 3.41
[H+] = ( T ^ O ) ( 0 . 1 0 0 ) => pH =
2.11
100.0 mL: [H+] = [^^)(0.100) => pH = 1.85
40
60
V a (mL)
108
Chapter 10
Initial mmol:
Final mmol:
0,500
0.336
0.164
—
—
Q.164
pH - pK\ + logjjj^j = 4.70 + log H ü = 5.01
10-27.
(a) Titration reactions:
H2NCH2CO¿ + H+ - • HjNCH2C02
Ve = 50.0 mL
+
HjNCH^Oj +H -H. H^CH 2 C0 2 H
Ve = 100.0 mL
At the second equivalence point, the formal concentration of
H3NCH2C02H is \J^j
(0.100) = 0.033 3 M
HÍNCH2C02H ^= HÎNCH2CO; + H+
0.0333 -x
x
x2
0.033 3 -x
x
= K]
Ä„
=
n m . . .
° 0 0 4 4 7 =>
*=1.02x 10 2 M =>pH = 1.99
(b) At Va = 90.0 mL, the approximation gives pH = pA| + log * CL = 2.35 +
log 4 = 1.75, which is lower than the correct value at 100.0 mL. The pH
calculated with the equation in Table 10-5 is 2.16, which is higher than the
equivalence point pH of 1.99.
At Va = 101.0 mL, the approximation gives [H+] = ( T ^ O W 100) = 6.62
x lO'4 M => pH = 3.18, which is higher than the correct value at 100.0 mL.
The pH calculated with the equation in Table 10-5 is 1.98, which is lower
than the equivalence point pH of 1.99.
10 28
- '
(a)
(b)
NH3
NH3
I
I
CHCH2CH2C02H + OH" ->
CHCH2CH2C02 + H 2 0
C02"
C0 2
V mL of glutamic acid will require QQ25
3
V
= 400
^ m L °f RbOH to reach
the equivalence point. The formal concentration of product will be
r/+4* 0QV J( 0 - 100 ) " 0.0200 M.
109
Acid-Base Titrations
V
(5.0* 10-5X1.1 * 10-|0)(0.Q20 0) + (5.Qx lQ-5)(l,Qx 1Q-14)
(5.0 x |0"5) +0.0200
• 7.42 x 10-8 M ^ pH = 7.13
10-29.
NH3
NH3
Cl ICI I 2 - ^ ^ - O i l + H+ ->
C0 2 "
CHCH2-(OVOH
C0 2 H
H2T
H3T+
One volume of tyrosine (0.010 0 M) requires 2.5 volumes of HCIO4 (0.004 00 M),
so the formal concentration of tyrosine at the equivalence point is
( 1 + ' 2 S J(0.0100 M) - 0.002 86 M. The pH is calculated from the acid
dissociation of H3T+.
H3T+ =*
0.002 86 -x
H2T + H+
x
x
0 , 0 0 2 8 6 - * = * ' - 3 . 9 x 1 0 - 3 =>
x = 0.001 92 M ^
10-30.
(a)
C2" + H + -» HC".
pH = 2.72
Ve = 20.0 mL. At the equivalence point, the formal
concentration of HC" is ( ^ ( O ^ O O ) = 0.0200 M.
m
_ ^pgM*
/(4,4* lQ-9)(l.82x IO-")(0.0200) + (4.4x 10-^(1.0 * 1Q-14)
V
(4.4 x 10 9 ) +0.020 0
= 2.86 x 10- 10 M => pH = 9.54
H3C+
(b)
0.0500 -x
r* H2C + H +
x
x
0.0500-JC " *»
F
002
= > x = 0.023M =>pH=1.64
pH = pA\ + log
F
=
[HC]
1.64 = 10.74 + log ^—L => ^—L = 7.9 x 10' 10
[HC"]
[HC]
110
10-31.
Chapter 10
The two values of pAa for oxalic acid are 1.250 and 4.266. At a pH of 4.40, the
QO4 bas not yet been half-neutralized.
Initial mmol:
Final mmol:
C2Q4- +
x
.v-16.0
pH = 4.40 = pK2 + log
H+
16.0
—
-»
HC2Q4
16.0
[ClOJ]
jc-16.0
[HC2Cr]
= 4.266 + l o g - j ^ ô —
=>x = 37.8 mmol of K 2 C 2 0 4 = 6.28 g
10-32.
Neutral alanine is designated FIA.
HA
Initial mmol:
Final mmol:
PH
"
PKl
+
1.2605
0.7445
'°8 i S ;
+
OH"
->
0.516
—
9
"
A"
+
H2Q
0.516
= P
*2
+ t
0
^
'
S
*
P^2 •
' M
10-33.
A Gran plot allows us to find the equivalence point by extrapolating from points
measured prior to the equivalence point.
10-34.
It is evident from the following tabic of data that the end point is near 23.4 mL,
where the derivative dpHldVb is greatest, A graph of F610-PH versus Vb follows.
The points from 21.01 to 23.30 mL were fit by the method of least squares to give
the equation shown in the graph. The intercept is found by setting >> = 0 in the
equation, giving x = Fe = 23.39 mL.
Fb(mL)
21.01
21.10
21.13
21.20
21.30
21.41
21.51
21.61
21.77
21.93
Pb10-PH
15.22 x 10-6
14.94
14.62
14.33
13.75
12.90
12.10
11.61
10.42
9.35
Kb(mL)
22.10
22.27
22.37
22.48
22.57
22.70
22.76
22.80
22.85
22,91
Vb 10"PH
8.40 x lO"6
7.37
6.60
5.91
5.29
4.53
4.14
3.78
3.46
3.16
Kb(mL)
22,97
23.01
23.11
23.17
23.21
23.30
23.32
23.40
23.46
23.55
Vb 10"PH
2.76 > 10-"
2.41
1.79
1.46
1.16
0.75
0.42
0.12
0.01
0.003
Acid-Base Titrations
1.5E-05
y = _6.4775 x 106x + 1.5153 x W
1.0E-05
i
o
.o
>
Intercept
= 23.39 mL
5.0E-06
0.0E+00 I i ' i i I i i • ' I •
21.0
21.5
22.0
22.5
23.0
23.5
V b (mL)
30000 i ' ' ' '
10-35.
20000
>
Ï
^^^^^l i i i i |
End point
10.727 mL
10000
-10000
CNJ
-20000
1
^_I_I_I^_I_I_
•••' *-•• **••*• * •
-30000 • - "
10.69 10.70 10.71 10.72 10.73 10.74 10.75 10.76
Volume (mL)
Calculations are shown in the spreadsheet:
24.0
112
Chapter 10
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
10-36.
A
B
C
E
I
D
I
F
Derivatives in a titration curve
DEda
1st derivative
2nd derivative
ApH/AmL
mL NaOH
pH
mL
A(ApH/AmL)
mL
10.679
7.643
AmL
10.6875
-11.5
10.696
7.447
10.6960
-553.6
-20.9
10.7045
10.713
7.091
10.7108
-2234.7
10.7170
-48.9
10.721
6.700
10.7200
-11770.8
10.7230
-119.5
6.222
10.725
10.7250
-21375.0
10.7270
-205.0
10.729
5.402
10.7290
25687.5
-102.2
10.7310
4.993
10.733
12411.1
10.7333
10.7355
-46.4
10.738
4.761
10.7398
2351.0
10.7440
-26.4
10.750
4.444
10.7508
885.2
10.7575
-14.5
4.227
10.765
Representative formulas:
C5 = (A6+A4)/2
E6 = (C7+C5)/2
D5 = (B6-B4)/(A6-A4)
F6 = (D7-D5V(C7-C5)
The quotient [HIn]/[In"] changes from 10:1 when pH = pATnin - 1 to 1:10 when
pH = pA^iiin + 1. This change is generally sufficient to cause a complete color
change.
10-37.
The indicator has its acidic color when pH = pA+iin - 1 because Hin is the
dominant species. The indicator has its basic color when pH • pK}\\n + 1 because
In" is the dominant species. The color changes from the acidic color to the
intermediate color to the basic color as the pH rises through the range pÂ+Un ~ 1 to
P^HIn + 1 • If the indicator is chosen correctly for the titration, this indicator pH
transition range coincides with the steep part of the titration curve. The color
change occurs near the equivalence point, which is the center of the steep portion
of the titration curve.
10-38.
The Henderson-Hasselbalch equation for the indicator, Hin, is
pH = pA^Hln + l°gfHIn]- l f w c k n o w Pellín and we measure TJTJ^T
spcctroscopically, then we can calculate the pH.
113
Acid-Base Titrations
10-39.
Strong acids, such as H 2 S04, HCl, HNO3, and HCIO4 have pKa < 0.
10-40.
yellow, green, blue
10-41.
(a)red(b) orange
(c) yellow
10-42.
(a)red(b) orange
(c) yellow
10-43.
No. When a weak acid is titrated with a strong base, the solution contains A" at
(d) red
the equivalence point. A solution of A" must have a pH above 7.
10-44.
(a) The titration reaction is F" + H + - » HF.
If V mL of NaF are used, Ve = 2V, since the concentration of HCIO4 is twice
as great as the concentration of NaF. The formal concentration of HF at the
( V \
equivalence point is
¡— (0.0300) - 0.020 0 M.
The pH is determined by the acid dissociation of HF.
HF
=*
H+
+ r
£
0.0200-JC
0.0200-JC
x
K
A a
-^
r =
i«xI0-3
=* X-1.MX 10
x
=> pH = 2.47
(b) The pH is so low that there would not be much (if any) break (inflection) in
the titration curve at the equivalence point. A sharp change in indicator color
will not be seen.
10-45.
(a) violet (red + blue)
10-46.
(a) N H 4 + ^ N H
0.010-JC
jr
3 +
(b) blue
H+
(c) yellow
njfc; - A * , - « • « »«11
x
=> pH = 5.62
(b)
One possible indicator is methyl red, using the yellow end point.
For a more complete analysis of this problem, wc could compute the titration
curve for a mixture of HCl and NH4+ For simplicity, we consider the mixture to
be a "diprotic" acid with K\ = 100 (i.e., a "strong" acid) and K2 = 5.7 x 10"10 for
the ammonium ion. We use the spreadsheet equation in Table 10-5 for titrating
H2A with strong base, taking C a = 0.01 M and Cb — 0.1 M. An indicator with a
color change in the range -4.5-7.0 would find the HCl end point without titrating
a significant amount of NH 4 .
114
Chapter 10
12
.---—~~~~*
11
T
10
Titration of HCl
Titration of NH»*
k 4
^
^
fc.
w*
w
j
r
1
5
10
15
volume of base (mL)
10-47.
Grams of cleaner titrated = (^ 10231+39466 j(10.231 g) = 0.900 3 g
mol HCl used = molNH 3 present =(0.014 22 L)(0,1063 M) = 1.512mmol
1.512 mmol NH3 = 25.74 mgNH 3
2.574» lO'2 e x
wt%NH 3 =
0,9003 g
' 0 0 = 2.859%
10-48.
Alkalinity is the capacity of natural water to react with H + to reach pH 4.5, which
2
is the second equivalence point in the titration of carbonate (CO 3") with H + .
2-
Alkalinity measures [OH"] + [CO 3 ] + [HCO3] plus any other bases that arc
present. Bromocresol green is blue above pH 5.4 and yellow below pH 3.8. It
will be at its green end-point color between these two pH values, which
approximates pH 4,5,
10-49.
Tris(hydroxymelhyl) aminomethane (H2NC(CH2OH)3), mercuric oxide (HgO),
sodium carbonate (Na2C03), and borax (NaB407 • 10H2O) can be used to
standardize HCl. Potassium acid phthalate (H02C-C6H4-CO¿K+), HCl azeotrope,
potassium hydrogen iodate (KH(I03)2), sulfosalicylic acid double salt
(C7H5S06KC7H4S06K2), and sulfamic acid i + ^ N S O j ) can be used to
standardize NaOH.
115
Acid-Base Titrations
10-50.
The greater the equivalent mass, the more primary standard is required. There is
less relative error in weighing a large mass of reagent than a small mass.
10-51.
Potassium acid phthalate is dried at 105* and weighed accurately into a flask. It is
titrated with NaOH, using a pH electrode or Phenolphthalein to observe the end
point.
10-52.
4 963
Grams of tris titrated = n 023'+99 367) ^
023
Concentration of HNO3 = 5 262 K solution
„ ^ f ,
10-53.
True mass = m =
?
1
=
^
= 0 0 5 0 5 7
=
0.4175 mmol
0.079 34 mol/kg solution
0.0012^
ofjoTYi
~ 1.023s g
I " 1.33 J
Failure to account for buoyancy introduces a systematic error of
100 x (L023g - 1.023) / 1.023 = 0.08% in the calculated molarity of HCl.
The true mass is higher than the measured mass of Tris, so the calculated HCl
molarity is too low.
10-54.
The mmoles of HgO in 0.194 7 g = 0.898 9, which will make 1.798 mmol of OH"
by reaction with Br" plus H 2 0. HCl molarity = 1.798 mmol/17.98 mL = 0.100 0 M.
10-55.
30 mL of 0.05 M OH" = 1.5 mmol = 0.31 g of potassium acid phthalate.
10-56.
(a) From a graph of weight percent vs pressure,
HCl = 20.254% when P = 746 Torr.
(b) We need 0.100 00 mole of HCl = 3.646 1 g
3.6461 g HCl
. . .
nnt
=
0.202 54 g HCl/g solution
18.0019 g of solution.
The mass required (weighed in air) is given by Equation 2-1.
(
0.0012^
(18.0019)[l-1Wj
m
'-
10-57.
( acorn
L1" 8.0 J
"
17985
ê
(a) For a rectangular distribution of uncertainty in atomic mass, divide the
uncertainly listed in the periodic table by ^ 3 to find the standard uncertainty:
116
Chapter 10
C: 12.0107 ± 0.0008A/3 = 12.0107 + 0.0004«
H: 1.007 94 + 0.00007/^3 - 1.00794 +0.000 040
O: 15.999 4 ± 0.000 SAß - 15.9994 +0.000 17
K: 39.098 3 ± 0.000 1A/3 = 39.098 3 ± 0.000 0S8
8C:
8(12.0107 +0.000 46)
5H:
5(1.00794 +0.00004o)
40:
4(15.9994 +0.000 17)
1K:
1(39.098 3 +0.0000s«)
C8H5O4K:
96.085 6 + 0.003 7
-
5.039 7 + 0.000 20
-
63.997 6 ± 0.000 69
3tJ.0t>X 3±O.0000 5g
204.221 2 + ?
Uncertainty = ^0.003 7 2 + 0.000 202 + 0.000 69 2 + 0.000 0582 = 0.003 8
Answer: 204.221 ± 0.004 g/mol
(b) For a rectangular distribution, divide the stated uncertainty by -\ß to find the
standard uncertainty. Purity = 1.000 00 ± 0.000 05A/3 = 1.000 00 ± 0.000 03
10-58.
5.00mLof0.033 6MHCl = 0.1680 mmol. 6.34 mL of 0.0100 M NaOH
= 0.0634 mmol. HCl consumed by NH3 = 0.1680-0.063 4 -0.104 6 mmol =
1.465 mg of nitrogen. 256 pL of protein solution contains 9.702 mg protein.
1.465 mg of N/9.702 mg protein =15.1 wt%.
10-59.
When an acid that is stronger than H30 + is added to H 2 0, it reacts to give H30 +
and is "leveled" to the strength of H30 + . Similarly, bases stronger than OH" are
leveled to the strength of OH".
10-60.
Methanol and cthanol have nearly the same acidity as water. Both of the
following equilibria arc driven to the right because of the high concentration of
H 2 0:
CH3O" + H 2 0 - • CH3OH + OH"
CH 3 CH 2 0" + H 2 0 -+ CH3CH2OH + OH'
10-61.
(a) In acetic acid, strong acids are not leveled to the strength of CH3C02H2 .
Therefore, very weak bases can be titrated in acetic acid.
(b) If tetrabutylammonium hydroxide were added to an acetic acid solution, most
of the hydroxide would react with acetic acid instead of analyte. However,
OH" will not react with pyridine, so this solvent would be suitable.
10-62.
Sodium amide and phenyl lithium arc stronger bases than OH-. Each reacts with
117
Acid-Base Titrations
H 2 0 to give OH":
NH¿ + H 2 0 — NH3 + OH"
dyHl + H 2 0 — C 6 H 6 + OH"
10-63.
The reaction of pyridine with acid is (T
J N + H* r= \ C ) NH+
Methanol is less polar than water. If methanol is added to the aqueous solution,
the neutral pyridine molecule will tend to be favored over the protonated
pyridinium cation. It will take a higher concentration of acid (a lower pH) to
protonatc pyridine in the mixed solvent. pKa for pyridinium ion is lowered when
methanol is added to the solution.
10-64.
Titration reaction: K+HP" + Na+OH" - • K+Na+P2" + H 2 0
Begin with CaVa moles of K+HP" and add CbVb moles of NaOH
CbVb
Fraction of titration = A = r v
Ca'a
Charge balance: [H+] + [Na+] + [K+] = [HP"] + 2[P2"] + [OH"]
Substitutions:
[K+] = vV ,W
v.
a
b
+
[Na
^ a J] •
[HP"] - ct„p- 7 7 - T ^
[P2-] = a P 2- -p—p¿
Putting these expressions into the charge balance gives
CbVb
CaVa
CaVa
[H+] +
+
y^Vb
v^Vb
= aw
'y^K
+ 2ap2
Va+Vb
CaFa
T^PTb + [°H"i
Multiply by Va+ Vb and collect terms:
[H+]F-a + [H+] Vb + CbVb + CaVa = a H P -C a ^ a + 2a p 2-C a P a + [OH"]ra + [OH"]Kb
Va ([H+] + C a - a H P -C a - 2ap2-C a - [OH"]) - Vb([OK] - [H+] - Cb)
Vb
F
a H P -C a •• 2q p 2-C a - C a - [H+] + [OH"]
C b + [H + ]-[OH"]
l/C a
Multiply both sides by JjrTa
CbVb
C
a*a
„ , . ÍH'l-fOH"!
QHr + 2 a p 2 - - l ca
,+
rH + ]-[OH"l
cb
118
Chapter 10
12
10-65.
T
—
'
—
i
—
«
—
„ o.
pK=10
i
—
•
m
•
—
•
"
10
B r
.
X
a.
•
•
pK = 6
o
*
pK = 4
n
4 :
pK = 2
2ir
,•
Strong acid
0
* • *
4
6
8
10 12
Volume of base (mL)
2
12
10-66.
14
16
T—3H,
11
20 mM HA
10
2mMHA
5°
+
9
S 8
0.2 mM HA
-J
L
J
i
I
i
L
4
6
8
10
Volume of base (mL)
n
A
10-67.
I
2
3
4
5
6
7
8
9
to
n
12
13
14
C
1)
J__i_
12
14
*
F.lYect of pKb in the titration of weak base with strong acid
pH
Ca =
0.1
Cb =
0.02
1H + 1
2.00 1 00E-O2
2.90 1.26E-03
3.50 3.16E-04
Vb-
•I.íIM 1.00E-04
50
K(BH+) =
IE-04
Kw-
4.50 3.I6E-05
6.00 1-00R-06
8,15 7.08E-09
n;-M
C4 = 10A-B4
D4-SAS12/C4
»
G
Alpha(l3H+)
Phi
Va (mL)
ÍOH-1
1.00L-I2
9.90E-01 1.66B+00
16.557
7.94E-12
9.26E-01
l.OOE+00
10.020
3.I6E-1I
7.60E-01
7.78E-01
7.780
I.00E-10
5.055
5.00E-01
5.06E-01
3.16E-10
2.419
2.40E-01
2.42E-01
1.00E-08
9.95E-03
0.100
9.90B-03
1.4IE-06
7.08E-05
5.17E-07
0.000
E4 = C4/(C4 +SAS10)
F4 = (E4+(C4-D4)/$A$6V( -(C4-D4V$y « 4 )
Ü4 = K4*$AS6*SA$8/5AS4
119
Acid-Base Titrations
14
12
T—•—I • I
' I '
Strong base
Ù D
*D
pK(base) = 2*
><>
pK(base) = 4
pK(base) = 6
" •
•
. .
pK(base) = 8
0
pK(base) = 10
2
4
6
• - • • • •
I
8
I
i
10
12
L
,:' I
14
.
16
Volume of acid (mL)
10-68.
(a)
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
A
B
C
D
Titrating weak acid with weak base
Cb =
pH
0.1
Ca =
0.02
Va =
50
Ka =
1 E-04
2.86
3.00
4.00
5.00
6. no
7.00
8.00
E
F
H
G
Vb(mL)
fOH-1
Alpha(A-) Alpha(BH+) Phi
IH+1
-0.01
6.76E-02
1.00E+00
-1.4E-03
1.4E-03 7.2E-12
0.41
4.1 E-02
9.09E-02
1.00E+00
1.0E-03 1.0E-1I
4.9E-01
4.95
I.00E+00
1.0E-04 1.0E-10
5.00E-01
9.09
9.IE-01
I.0E-09
9.09E-01
9.99E-01
1.0E-05
10.00
9.90E-01
1.0E+00
9.90E-0I
1.0E-06 1.0E-08
9.09E-01
10.99
1.1E+00
9.99E-0I
l.OL-07 I.0E-07
2.0E+00
20.00
1.0E-08 I.OE-06 I.00E+00
5.00E-0I
Kw =
1E-14
Kb1E-06
K(BH+) =
1E-08
D4 = $A$12/C4
A16 = $A3Î12/SASI4
E4 -SASI0/(C4+$A$10)
C4= I0A-B4
F4 = C4/(C4+$AS16)
G4 = (E4-(C4-D4VSA$6y(F4+(C4-D4V$A$4)
H4 - G4*SA$6*$AS8/5A$4
1
120
Chapter 10
11
10
pK b = 3
g
f
pKb=6
8
7
6
pK b = 9
5
4
3
2
0
5
10
15
20
Volume of base (mL)
(b)
HA
K& = 1.75 x 10-5
+
A" + BH+
B
Kb=l.S9x 10-10
Fa = 212 mL
ä : B H + = 6.28 * I0-5
C a = 0.200M
Kb = 325mL
Cb = 0.0500 M
To find the equilibrium constant wc write
HA
^
A" + H +
Ka
H+ + B
^
BH*
l//fBH+
A" + Bll+
K=Ka/KBH+ = 0.219
HA + B ^
A pH of 4.16 gives Vb = 325.0 mL in the following spreadsheet:
1
2
3
4
5
6
7
8
9
III
11
12
13
14
15
16
A
B
C
1)
Mixing acetic acid and sodium benzoatc
Cb =
0.05
Ca
Va =
pH
4.00
4.2
0.2
4.1
4.15
212 4.1598
Ka1 750E-05
Kw =
I.E-14
Kb =
I.592E-10
K(BH+) =
6.281E-05
E
F
G
El
IOH-1
Alpha(A-) Alpha(BH+) Phi
[H+l
Vb (mL)
I.OE-04
I.OE-tO
1.49E-01
6.14E-01
2.4E-0I
204.28
6.3 E-05
1.6E-10 2.17E-01
4.3E-01
5.0IE-0I
365.98
I.3E-I0
1.8IE-01
7.9E-05
5.58E-01
272.78
3.2E-01
I.4E-I0
7.1 E-05
I.98E-01
5.30E-01
315.79
3.7E-01
I.4E-I0
6.90-05
2.02E-0I
3.8E-01
5.24E-01
325.03
AI6 = $AS12/$AS14
C4= l O * - ^
D4 = $ASI2/C4
E4 = $A$10/(C4+$A$10)
F4 = C4/(C4+$ASI6)
G4 = (E4-(C4-D4)/SAS6V(F4+(C4-D4)/SAS4)
114 = G4*$A$6*$AS8/$AS4
121
Acid-Base Titrations
10-69.
H
E
G
D
F
B
C
A
1 1 itratin« diprotic acid with strong base
2
1
Vb (mL)
,11
OH-1
Aipiui(HA-) Alpha(A2-) Phi
3 Cb
H+l
5.00E-07
5.0E-05
O.OOC
7.3E-I2
6.83E-02
1.4E-03
4
0.1 2.865
4 946
4.9E-0I
5.00E-05
1.0F.-04 1.0E-IO
5.00E-01
4.0O
5 Ca =
9.999
9.801-03
1.0E+00
9.80E-01
1.0E-06 1 .OE-08
0.02
6.00
6
15.000
1.5E+0C
N.lll
5.00E-01
5.00E-01
1.0E-08 1.0E-06
7 Va =
19.971
2.0E+0C
1.0E-04
9.90E-03
9.90E-01
10.1
1.0E-1Ö
5C
8
27.777
1.00E-04
I.00E+00
2.8E+0C
l.OE-12 1.0E-O2
12.0
9 KwIE-14
10
D4 = $AS12/C4
C4= 10A-B4
10 KlE4 = C4*SA$l2/(C4A2+C4*SA$l2tSASI2*SA$l4)
1E-4
12
F4 = $A$12*$A$14/(C4A2+C4*SA$I2<SA$12*$A$14)
13 K2 =
< 14 i E4 - 2*F4-(C4-D4)/SA$6V( 1 t(C4-D4)/$A$4)
I.L-08
14
H4 = G4*$AS6*SA$8/SA$4
15
(b)
r
0
5
«•
10
15
20
Volume of base (mL)
25
30
122
Chapter 10
10-70.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
A
B
C
Titrating nicotine with strong acid
pH
Cb =
0.1
1.75
2.00
3.00
4.00
5.00
6.00
7.00
8.00
9.00
10.00
Ca
0.1
Vb
10
Kw
l.E-14
KB1 7.079E-7
KB2 =
I.41E-I1
KAI =
7.077F.-4
KA2I.4I3E-Í
1(1.42
D
E
F
G
H
IOH-1
Alpha(BH2) Alpha(BH) Phi
IH+1
Va (mL)
I.8E-02 5.6E-I3
9.62E-01
3.83E-02
2.6E+00
26.023
I.0E-02 LOE-12
9.34E-01
6.61 E-02 2.3E+00
22.599
1.0F.-03 1.0E-I1
5.86E-01
4.14E-0I
1.6E+00
16.117
1.0F.-04 LOE-10
1.24E-01
8.76E-01
I.1E+00
11.258
I.0E-O5 1.0E-09
1-39E-02
9.85E-01
1.0E+00
10.127
1.0E-06 1.0E-08
1 39E-03
9.85E-01
9.9E-01
9.875
I.0E-O7 1 .OE-07
I.24E-04
8.76E-01
8.8E-01
8.764
I.0E-08 1 .OE-06
4.14E-01
5.86E-06
4.IE-01
4.145
1.0E-09 I.OE-05
6.61 E-02
9.34E-08
6.6E-02
0.660
LOE-10 I.OE-04
9.93 E-10
0.1)60
7.03E-03
6.OE-03
3.8E-11 2.6E-04
1.44E-10
2.68E-03
5.4E-05
0.001
C4= 10^-64
D4 = $A$]2/C4
E4 = C4«C4/(C4A2+C4*$A$16+$ASI6*$A$18)
F4»C4*$AS16/(C4A2-K:4*$ASI6+$A$16»$A$I8)
G4 = (F4+2»E4-KC4-D4)/$AS4V(I-(C4-D4V$A$6)
114 • G4*$A$4*$A$8/$AS6
10-71.
B
Titrating HiA with NaOH
pH
<v
0.1
1.89
2.00
1.29E-02
1.00E-02
0.02
3.00
4.00
1.00E-03
1.00E-04
50
5.00
6,00
1.00E-05
V.=
K« :
1E-14
10
11
12 2.51E-02
13
14 1.07E-06
15 K.,=
16 5.25E-10
17 p K , j
18
19
PKï =
5.97
!
21 pKa
22
1.00E-06
1.00E-07Í
1.00E-08
7.00
8.00
9.00;
1.O0E-09
10.00
11.00
12.00
1.00E-10
1.O0E-11
1.O0E-12
C4
Alp b a(H a A) Alpha{HA'")
[OH]
7.76E-13
661E-01
5.50E-05
1.00E-12
M5E-01
7.66E-05
9.61E-01
1.00E-11
1.03E-03
1.00E-10
9.86E-01
1.06E-02
Alpha(A s )
Phi
Vb (mL)
2.24E-12
4.02E-12
1.50E-02
1.96E-01
0.150
1.958
5.40E-10
5.54E-08
9.04E-01
1.00E+00
9.037
10.006
10.958
1.00E-O9
1 .OOE-Oß
9.03E-01
4.83E-01
9.67E-02
5.17E-01
5.08E-06
2.71 E-04
1.10E+00
1.52E+0O
1.00E-07
9.10E-O1
1 OOE-06
8.5OE-02
8.79E-03
9.42E-01
4.78E-03
4.94E-02
1.92E+00
2.04E+00
1.00E-05
1.0OE-04
6.12E-04
1.49E-05:
6.55E-01
1.60E-01
3.44E-01
8.4QE-01
2.34E+00
2.85E+00
23.441
1.00E-03
1.Û0E-02
1.75E-07!
1.77E-09
1.87E-02
1.90E-03
9.81E-01
3.06E+OQ
3.89E+00
30.619
38.868
9.98E-01
15.176
19.198
20.407
28.478
10*-B4
L>4
1.60
20
Kl
9.28
$A$10/C4
E4 = $C4 A 2*$A$12/($C4 A 3+$C4 A 2*$A$12+$C4*$A$12*$A$14+$A$12 , $A$14'$A$16)
F4 = $C4*$A$12'$A$14/($C4 A 3+$C4*2*$A$12+$C4*SA$12*$A$14+$A$12*$A$ 14*$A$16
G4^$A$12 T $A$14 > $A$16/($C4 ft 3*$C4 A 2*$AS12*$C4 , $A$12-$A$14^tr%$12 , $AS14*$A$16)
,H4 = (E4+2'F4+3*G4-(C4-D4)/$A$6)/(1-f{C4-D4)/$A$4)
14 - H4'$A$6'SA$8/$A$4
123
Acid-Base Titrations
Vb mL
QBH+ + 2aßH22+ + 3aBH33 + + 4asH44+ +
10-72.
4» = C V
b b
[H + l - [OH-1
qj
nri-iOHca
A
1
B
C
D
H
F
[0*1
a(BH-)
a(BH, î 4 >
G
H
1
J
atBH,»*)
a(BH/*)
Phi
V„ (mL)
Tit rat ing Tet rabaslc B wil h H*
2
3
4
Q,=
pH
0.02
5 <* =
0.1
6
7
8
v fc =
50
9 Kw =
1E-14
10
11 K.i =
12
1.26E-01
13 K,j =
5.25E-03
14
15 K * 16
2.00E-07
17 K« =
18
3.98E-10
pK.
=
19
20
0.90
1 3E-11
1.OE-10
7.9E-04
3,1 E-02
1.9E-06
4.6E-15
10.0
1.0E-04
2.0E-01
1.0E-04
1.9E-12
4.6E-25
1.5E-21
-0.009
0.196
-0.090
1 957
9.0
8.0
1.0E-09
1.0E-08
1.0E-05
1.OE-06
7.1E-01
92E-01
3.6E-03
4.6 E-02
6.8E-10
8 8E-0B
5.4E-18
7.0E-15
0719
1.009
10.094
7.0
6.0
1.0E-07
1 OE-06
1.0E-07
1.0E-08
6.6E-01
1.7E-01
3.3E-01
6.3E-06
83E-01
1.6&04
5.0E-12
1.3E-09
1.330
1.834
5.0
4.0
1.0 E-05
1.0E-04
1.0E-09
10E-10
2.0 E-02
9.8E-01
2.0E-03
9.8E-01
1.9E-03
1.9 E-02
1.5E-07
1.5E-05
1.983
2.024
20.238
3.0
2.0
1.0E-03
1.0E-02
1 .OE-11
1.0E-12
1 7E-04
6.5E-06
8.4E-01
3.3E-01
1.6E-01
6.2E-01
1.3E-03
5.0E-02
2.235
3.580
22 345
35.804
1.7
2.0E-02
5.0E-13
1.9&06
1.9E-01
7.0E-01
1.1E-01
4.902
49022
10.9
C12 = 1 0 * - A 20
C4 = 10 A -B4
D4 = $A$10/C4
Denominalor = ($C4 A 4 + $C4 A 3*$A$12«-$C4 A 2'$A$12'$A$14
|+$C4*$A$12*$A$14*$A$16+$A$12*$A$14*$A$16*$AÎ 18}
E4 = $C4-$A$12*$A$14*$A$16/Denominator
F4 = SC4 A 2*$A$12*$A$14/Denominator
21 PK,=
22
[H*l
2.28
G4 = $C4 A 3*$A$12//Denominator
23 PK,. =
6.70
24
H4 = $C4 A 4/Denominator
M = ÍE4+2*F4+3*G4+4*H4+(C4-D4)r*$AS4) r '(1-{C4-D4)r'$A$6)
25 pK, =
9.40
26
J4 • W*$A$4*$A$8/$A$6
7 193
13.303
18.338
19830
124
Chapter 10
CL
10-73.
Am
= e In -[m - ] (1.00) => [In"] = ^ " ^
= 2.37 * 10^M
Since the indicator was diluted with KOH solution, the formal concentration of
indicator is 0.700 x 10"5M.
[Hin] = 7.00 x 10-6-2.37 x 10"6 - 4.63 x 10-6 M
pH = pÂ-in + log 7 ^
= 7.95 + l o g | H = 7.66
Call benzene- 1,2,3-tricarboxylic acid H3A, with pA'i = 2.88, pA2 = 4.75, and pKy
= 7.13. Since the pH is 7.66, the main species is A 3- and the second main species
is HA2". Enough KOH to react with H3A and H2A" must have been added, and
there is enough KOH to react with part of the HA 2 '.
HA 2 ' +
OH"
-»
A3-
+
H20
Initial mmol: 1.00
Final mmol: 1.00-j
pH = pA*3 + log JA!±
[HA2-]
7.66 = 7.13 + log-r^fj-
x - 0.772 mmol of OH"
1
The total KOH added is 2.772 mmol. The molarity is
T7
wtw*
"¿JMÏmL
1
=
°-139
M
-
125
Acid-Base Titrations
10-74.
The pH of the solution is 7.50, and the total concentration of indicator is
5.00 x 10"5 M. At pH 7.50, there is a negligible amount of H2In, since
pA'i = 1.00. We can write
[Hin"] + [In2"] = 5.0 x lf>5
[In2']
7.50 - 7.95 + tog 5 0 0 / , ' 0 " ! 1 . [ , n 2 - ] -
P * l - »•" " ' ° - i M
[Hin] - 3.69 x 10-5 M
^435 = e435[HIn"] + 8435[ln2"]
= (1.80 x 104)(3.69x 10-5)+ (1.15 x io4)(l.31 x 1Q-5) = 0.815
CHAPTER 11
EDTA TITRATIONS
11-1.
The chelate effect is the observation that multidentate ligands form more stable
metal complexes than do similar, monodentate ligands.
11-2.
ay4- gives the fraction of all free EDTA in the form Y 4 \
(a)
At pH 3.50:
10-0.0 IQ-1.5 lQ-2.00! 0-2.69) Q-o.13 jQ-10,37
" "= (10-3-50)6+(10-3.50)510-0.0-f„.+ | 0 0.0| 0 -1.5... 10-10.37
ttY4
(b)
(a)
"
==
10-0.0l0-l.5l0-2.00l0-2.69iQ-6.l3l0-l0.37
(10- 0'50)6 + (lo-l0.50)5io-0.0+_+io-O.OlO-L5.„iO-10.37
"
|
=
°-57
AY = aY4-ATf = 0.041 x io«79 = 2 .5 x \Ql
Mg 2+ + EDTA ^
(b)
x
005
11-4.
X 10 10
At pH 10.50:
aY4
11-3.
== 2J
X
X°2~
MgY2-
x
0.050 -x
= 2.5 x 107 -> r Mg 2+] = 4.5 x 10-5
M
[Ca2+] = 10"9-00 M, so essentially all calcium in solution is CaY2".
t C a Y 2 "i =(200.12 g)mo5|H0.500L) = 0.019 49 M
A7f - Î0 041Ï1010.65)
=
í C a Y 2 "l 2+ wl
^- X»0
)
[EDTA][Ca ]
0 949x10" 2 )
[EDTA] (10-9.00)
=> [EDTA] = 0.010 6 M
Total EDTA needed = mol CaY2" + mol free EDTA
= (0.019 49 M) (0.500 L) + (0.010 6 M) (0.500 L) - 0.0150 4 mol
= 5.60gNa 2 EDTA-2H 2 O
.co2"
11-5.
H02C
"OfctT
NÍ
^-"'H ^ - ^
»N
HsDTI'A
X0 2 H
>COi
Neutral H5DTPA has 2 carboxylic acid protons and 3 ammonium protons. We
are not given the pA"a values, but, by analogy with EDTA, wc expect carboxyl
pA'a values to be below ~3 and ammonium pA'a values to be above - 6 . At pH 14,
wc expect all acidic protons of DTPA to be dissociated, so the predominant
126
127
EDTA Titrations
species will be DTPA5". At pH 3-4, nitrogen should be protonated, but carboxyl
groups should be deprotonatcd. The predominant species is probably H3DTPA2-.
For HSO4, pA'a = 2.0. At pH 14 and at pH 3, sulfate is in the form SO|".
At pH 14, DTPA5" is apparently a strong enough ligand to chelate Ba2+ and
dissolve BaS04(s). At pH 3-4, H3DTPA2- is not a strong enough ligand to
dissolve BaS04(s). An equivalent statement is that H+ at a concentration of
ÎO^-IO"4 M competes with Ba2+ for binding sites on DTPA, but H+ at a
concentration of 10-'4 M does not compete with Ba2+ for binding sites on DTPA.
Now that you have seen my reasoning, I'll provide some more information.
The pA'a values for DTPA, beginning with the fully protonated HgDTPA3+, are
HgDTPA 3 *
H7DTPA2+
H6DTPA+
H5DTPA
pA'i =-0.1
pA-2 = 0.7
pA-3 = 1.6
pA"4 = 2.0
C02H
C02H
C02H
C0 2 H
H4DTPAH 3 DTPA 2 H2DTPA 3 H DTPA4-
pA-5 = 2.7
pA:6 = 4.3
pA7 = 8.6
pA:8=10.5
C02H
NH+
NH+
NH+
As pH is lowered from 14, the three nitrogen atoms are 50% protonated at pi I
10.5, 8.6, and 4.3. The third nitrogen atom is not quite fully protonated at pi I 34. The predominant species is H3DTPA2-, as I guessed correctly. The species
H4DTPA- and H2DTPA3' are also present to some extent in the pH range 3-4.
11-6.
(a)
mmol EDTA = mmol M"+
(^(0.0500 M) » (100.0mL)(0.050 0M) => Ve= 100.0 mL
(b)
[M"+]= Q)
•
fraction
remaining
(0.0500M)- (jfjj = 0.0167 M
original
concentration
dilution
factor
(c) 0.041 (Table 11-1)
(d) Af = (0.041X101200) - 4.1 x 101°
(e)
[MY» - 4 ] - (0.050 0 M) {^j
(f)
[EDTA] = (0.0500 M) M^fJ = 2.38 x 10"3 M
= 0.025 0 M
[MY" 4 ] = (0.050 O M ) ^ ) = 2.38 x 10"2M
iMY*-41 =
(2-38
*1Q-2)
=4 , * lU0 ' 0 ^ [[M
« M - 2 4 , lO-'OM
+
[M«'][EDTA] " [M» ](2.38^10-3) 4 J
'
= * M J ¿A
128
11-7.
Chapter 11
Co 2+ + EDTA =* CoY2" c^t-ATf = (1.8 x lO^XlO 1645 ) = 5.1 x ] 0 "
fO.020 26 VF\
mm
^ - (25.00) ( O Q 3 8 5 5 M j = 13.14 mL
(a) 12.00 mL: [Co2*] = [l3% ^
(0.020 26 M) ( f f f j )
= 1.19 x 10-3 M => pCo 2+ = 2.93
(b)
Vc: Formal concentration of CoY2" is [w^
Co 2+ + EDTA
X
(0.020 26 M) = 1.33 x 10'2 M
CoY2-
==
1.33XIO-2-JC
x
2
1.33 x IQ- -*
^2
= aY4-A"f => x = 1.6 x ]0- 7 M => pCo 2+ - 6.79
14.00 mL: Formal concentration of CoY2" is k ^ ^ J (0.020 26 M)
(c)
= 1.30x10-2 M
Formal concentration of EDTA is Í
r^
[C
11-8.
rCoY2']
2+1
°
] =
3900
J (°038
55 M
) = 8-50
x
10"4 M
1.30x10-2
=
]^ÏATK]
8.50xi(H(5.1xioii) =
=> pCo2+ = 10.52
3 0 X 10 n M
-
Titration reaction: Mn 2+ + EDTA == MnY2*
Af=a Y 4-A:f = = (4.2 x 10-3)(101389) = 3.3 x 10' l
The equivalence point is 50.0 mL.
Sample calculations:
2+
20.0 mL: The fraction of Mn that has reacted is 2/5 and the fraction remaining
is 3/5.
[Mn 2+ ] = d J J I D ( 0 . 0 2 0 0 M ) ^ 4) = 6.67 x 10-3M => pMn2+ = 2.18
50.0 mL: The formal concentration of MnY2- is
[MnY2-] = ( j l j j j (0.0200 M) = 0.00667 M
Mn2+ + EDTA i=
x
x
MnY2"
0.006 67 -x
0.006 6 7 - *
"2
= aY4-ATf => x = 1.4 x 10-7 => pMn 2+ = 6.85
129
EDTA Titrations
60.0 mL: There are 10.0 mL of excess EDTA.
[EDTA] = (gjf)(0.0100 M) = 1.176 x I 0 3 M
[MnY2-] = \j¡¡fi\ (0.0200 M) = 5.88 x 10"3 M
2+
[Mn
]J = }^JA2'l
1
= 1.5 x lO!' => pMn2+ = 10.82
[EDTA]Ar
Volume (mL)
0
20.0
40.0
pMn2+
Volume
49.0
49.9
50.0
1.70
2.18
2.81
20
pMn2+
Volume
50.1
55.0
3.87
4.87
6.85
60.0
40
pMn2^
8.82
10.51
10.82
60
Volume of EDTA (mL)
11-9.
Titration reaction: Ca 2+ + EDTA ^ CaY2"
Af=a Y 4-Af = (0.30)(10'065) = ].3 4 x 10<0
The equivalence point is 50.0 mL. Sample calculations:
20.0 mL: The fraction of EDTA consumed is 2/5.
[EDTA] - ( | o : o ) ( 0 0 2 0 0 M ) ( 4 5 ! o )
=
0 0 0 6 6 7 M
2
°j^) (0.020 0 M) (Ü{j;J = 0.004 44 M
[CaY2-] = 50.
rCa2+] =
JCâYi
[EDTA]A-f
4.9 7 x 10-11 => pCa 2+ = 10.30
130
Chapter 11
50.0 mL: The formal concentration of CaY 2- is
[CaY2-] - \jffij
Ca 2+ + EDTA
(0.0200 M) = 0.00667 M
CaY2-
^-
0.006 67 -x
0.006 6 7 - *
= ccY4- Af => * = 7.O5 x 10-7 M - j , pCa 2 + = 6.15
50.1 mL: There is an excess of 0.1 mL of Ca 2+ .
[Ca2+] - ( ^ 7 ) (0.010 0M) = 1.33 x 10'5 M => pCa 2+ = 4.88
pCa 2+
Volume (mL)
0
20.0
40.0
Volume pCa 2 '
49.0
49.9
50.0
10.30
9.52
0
10
20
Volume pCa 2+
50.1
4.88
55.0
3.20
60.0
2.93
8.44
7.43
6.15
30
40
50
60
Volume of C a ^(mL)
11-10.
There is more V0 2 + than EDTA in this solution.
[VO 2 *] - ( | ¿ ! j (0.0100 M) = 3.34 x i0-5|vt
Í99Q\
[VOY 2 -] = (J^goj (0.0100 M) = 3.31 x lO"3 M
Af for VOY2- = JO1«7; pA- 6 forH 6 Y 2+ = 10.37; pH = 4.00
[Y4 ] =
"
[VV0°2+]2AV = l - 9 8 x l 0 - l 7 M
[HY3-] =
rH+
^ Y 4 " ] = 4.6 x 10-H M
EDTA Titrations
11-11.
C
I
D
A
B
E
2
Titration
of
V
mL
of
C
M
Cu
*
with
C(ligand)
M
EDTA
M
M
1
2
Phi
V{EDTA)
M
pM
3 c« =
0.000
0.000
1.00E-03
4
0.001
3.0
0.891
0.891
1.00E-04
4.0
5 VM =
10
6
7 C(ligand) =
0.01
8
9
10
11
12
13
14
15
16
17
18
19
K,' =
1.75E+12
«&*>
2.90E-07
K,=
6.0256E+18
5.0
6.0
7.0
8.0
9.0
10.0
11.0
12.0
12.3
1.00E-05
1.00E-06
1.00E-07
1.00E-08
0.989
0.999
1.000
1.000
0.989
0.999
1.000
1.000
1.00E-09
1.00E-10
1.00E-11
1.00E-12
1.001
1.006
1.057
1.572
1.001
1.006
1.057
1.572
5.01E-13
2.142
2.142
F
G
A10 = A12*A14
C4=10 A -B4
D4 = (1+$A$10»C4-(C4+C4*C4*$A$10)'$A$4 V(C4*$A$10+(C4+C4*C4*$A$10)/$A$8)
E4 = D4*SA34*$A$6/$A$8
14
Cu
12
10
S
8
Cd
6
0.0 0.2 0.4 0.6 0.8 1.0 M 1.4
Volume of EDTA (mL)
1.6 1.8 2.0
132
11-12.
Chapter 11
The spreadsheet below gives representative calculations for the pH 7.
D
E
c
F
1 Titration of 10 mL of 1 mM Ca2< with 1 mM EDTA vs pH
2 pH7
3 CM =
pM
M
Phi
V(ligand)
0.001
4
3.000
1.00E-03
0.000
0.000
5 VM =
3.250
5.62E-04
0.280
2.801
10
6
3.500
3.16E-04
0.520
5.196
7 C(ligand) =
3.750
1.78E-04
0.698
6.982
0.001
8
4.000
1.00E-04
0.819
8.186
9 K,' =
4.500
3.16E-05
9.404
0.940
1.70E+07
10
5.000
1.00E-05
0.986
9.859
11 u(Y<) =
5.500
3.16E-06
1.012
10.121
3.80E-04
12
6.000
1.Û0E-06
1.057
10.567
13 K,=
6.500
3.16E-07
1.185
11.855
14 4.4668E+10
7.000
1.00E-07
15.887
1.589
15 A10 = A12*A14
16 C4 = 10A-B4
17 D4 • (1+A$10*C4-(C4+C4*C!4VUB10)/A$4)/<C4 # A$10+(C4+C4*C4'A$10)/A$8)
18 E4 = D4*A$4*A$6/A$8
1
•! "
-"
A
|
B
|
I0
pH9
a
:•:
O
pH8
6
0
2
4
6
8
10
Volume of EDTA (mL)
12
14
133
EDTA Titrations
11-13.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
A
B
|
Titration of EDTA with metal
M
CM =
pM
0.08
V(ligand) =
50
C(ligand) =
0.04
14.640
12.844
12.418
12.066
11.640
10.860
6.910
2.978
2.301
Kf =
1.75E+12
«(Y 4 )
2.90E-07
K,=
6.0256E+18
D
C
2.29E-15
1.43E-13
3.82E-13
8.59E-13
2.29E-12
1.38E-11
1.23E-07
1.05E-03
5.00E-O3
E
Phi
0.004
0.200
0.400
0.600
0.800
0.960
0.100
5.004
10.007
15.004
20.003
24.005
1.000
1.040
1.200
25.000
25.999
30.000
F
G
VM
A10 = A12*A' 4
C4 = 10A-B4
D4 = (C4*$A3>10+(C4+C> *C4*$A$10)/$A$8)/(1 +SA$10*C4- C4+C4*C4'$A$10)/$A$4)
E4 = D4*$A$ 3*$A$6/$AS4
11-14.
An auxiliary complexing agent forms a weak complex with analyte ion, thereby
keeping it in solution without interfering with the EDTA titration. For example,
NH3 keeps Zn 2+ in solution at high pH, but is easily displaced by EDTA.
11-15.
(a)
ß 2 = KyK2 = ß|Ä2 => K2 = ßz/ßl = 10 3 - 63 / 10 2 2 3 = 101-40 = 25
(b)
ac u 2 + =
1
11-16.
1+ßl[L] + ß2[L]2
Cu 2+ + Y4" ^
CuY2"
=
1
1 + io 223 (0.100)+10 3 «(0.100) 2 =" ° ° 1 7
ATf = 10 18 - 78 = 6.O3 x 10' 8
a Y 4- = 0.81 atpH 11.00 (Table 11-1)
For Cu 2+ and NH3, Appendix I gives log ß] = 3.99, log ß 2 - 7.33, log ß3 = 10.06,
and log ß 4 = 12.03. Therefore, ßi =9.8 x 103, ß 2 = 2.1 x 107, ß 3 = 1.15 x 1010,
and ß 4 = 1.07 x 10' 2 .
0tCu2+
!_
= 9.2.1 x lO-13
1 + ßi(1.00) + ß 2 (l.00) 2 + ß3(1.00)3 + ß4(1.00)4
K{= a\4-K{=
4.88xl018
A"f = a Y 4- acu2+ Kf = 4.5, x 10°
Equivalence point = 50.00 mL
(a)
At 0 mL, the total concentration of copper is Ccu2+ = 0.001 00 M and
[Cu 2+ ] = ocu2+ Ccu2+ - 9.23 x 10-'6M => pCu 2+ = 15.03
134
Chapter 11
(b) At 1.00 mL, CCu2+ = ( ^ j j j ) (0.001 00 M) ( | y | | ) - 9.61 x I0"4 M
fraction
remaining
original
concentration
dilution
factor
[Cu2+] = acu 2+ Ccu 2+ = 8.87 x 10-16 M => pCu2+ - 15.05
At 45.00 mL, Cc„2+ - (¿J£$ (0.001 00) (~$
(c)
= 5.26 x l<)-5 M
[Cu2+] = acu2+CCu2+ = 5.0< x lO-«7 M => pCu2+ = 16.30
(d) At the equivalence point, we can write
Ccu2+ + EDTA
=*
CuY2"
( 50.00 \
*
(,7öäwJ<°- 00,00 >-*
0.000 5 0 0 - j
= ä f = 4.5| x lO« => JC = Ccu2+ = 1-04 x 10-5 M
^2
[Cu2í] = ocu 2 ' Ccu2+ = 9.62 x 10-|8M => pCu2+ = 17.02
(e)
Past the equivalence point at 55.00 mL, we can say
[EDTA] = ( 7 5 ^ 0 ) (0.001 00 M) = 4.76 x 10-5 M
[CuY2-] = [•(§£§)] (0.001 00 M) = 4.76 x 10"4 M
[CuY2'l
(4.76 x IP-4)
r
[Cu2+][EDTA] " [Cu2+] (4.76 - 10-5)
=> [Cu2+] = 2.05 x 10-18 M => pCu2+ = 17.69
¿ _
1117
"
M
W
[ML]
ßi[M][L]
p,[L]
2 =
" CM ~[M]{l+ß,[L] + ß2[L] } l+ß,[L] + ß2[L]2
[ML2] _
ß2[M][L]2
ß2[L]2
aML2
" CM - [M]{l+ß,[L] + ß2[L]2} : : I + ß,[L] + ß2[L]2
n
ML
For [L] - 0.100 M, ß| - 1.7 x lo 2 , and ß2 = 4.3 x lo 3 , we get
aML = 0.28 and CIML2 • 0.70.
Let T = transferrin
(b)
11-18.
(a) F^ + rSpeT
A
V>) l
*'={&
[FeaT] + [Fei,T]
[Fe,T]
[Fe|,T|
+
[Fe3+][T]
" [Fc3+][T] [Fe3+][T] ~ * l , + * l b
135
EDTA Titrations
J_
K2
=
[Fe3+]([FcaT] + [FcbT])
[Fe2T]
[Fe 3 1[Fc a T]
[Fe2T]
[Fc3+][FcbT]
1 1
[Fc2T]
' k2b k2a
,,
(c)
. .
P ^ l
tFe2T]
_ ÍEegT]
[Fe2T]
_
3+
3+
3+
*la*2b= [Fe ][T] [Fe ] P ^ T ]
[Fc^][T][Fe ]IEetrT] * l b / f 2 a
(d)
Substituting from Eq. (A) into Eq. (C) gives
[FeT]2
19.44 = ( i _ [ FcT ] _ [Fe2T]) [Fe2T]
(D)
Substituting from Eq. (B) into Eq. (D) gives
2
(0.8 -2[Fe 2 T])
19.44 --' {l _(0.8-2[Fe 2T]) - [FC2T]} [Fe2T]
solve
=>
quadratic
T1 = r i n T 7 ,
^C21 J VM i ' •>
equation
Using this value for [Fe2T] in Eqns. (A) and (B) gives [FeT] - 0.645 and
A|a
[FeaT]
[T] = 0.277 3. Now we also know that ¿— - Tp^TyT = 6.0, which tells us
that[Fe a T] = [ f | ) [FeT] = 0.553 2 and [FebT] = ( ^ ) [FeT] = 0.092 2.
Final result: [T] - 0.277t [FeaT] = 0.553, [FcbT] - 0.092, [Fe2T] = 0.077.
11-19.
In place of Equation 11-8, we write
™
u CHTA — M / . - n T M
Mfree + EDTA -
M(EDTA)
jf" -
fM(EPTA)]
Kf -
[M]free [ E D T A ]
where [M]free is the concentration of all metal not bound to EDTA. [EDTA J is
the concentration of all EDTA not bound to metal. The mass balances are
Metal:
[M] free + [M(EDTA)] =
y ^ + y ^
EDTA:
Cv DT A J^EDTA
[EDTA] + [M(EDTA)] =
yM+yEmA
These equations have the same form as the First three equations in Section 11-4,
with Kf replaced by K}, [M] replaced by [M]free, and [L] replaced by [EDTA].
The derivation therefore leads to Equation 11-11, with Kf replaced by Af, [M]
replaced by [M]ffee, and Q , replaced by CEDTA-
136
11-20.
Chapter 11
(a)
A
B
C
D
E
I
F
¿
1 Titration of 50 mL of 0.001 M Zn * with 0.001 M EDTA/pH 10 with NH3
2
pM
M
(MJtoi
4>
3 CM =
VEOTA
4
0.001
8.115
7.67E-09, 4.29E-04
0.400
19.9814
9.68E-13
5.41
E-08
1.000
50.0000
5 VM =
12.014
6
50
15.278 5.27E-16 2.95E-11!
1.200
59.9965
7 CEDTA 8
0.001
9 KTA10 = A12*A16*10AA14
10 1.70E+11 A12 = 1/(1 +A20*A18+B2!0*A18A2+C20*A18A3+D20*A18A4)
11 a(ZrP) =
12
^^^^^^^—
1.79E-05 C4 = 10A-B4
13 logK,=
D4 = C4/$A$12
14
16.5 E4 = (1+$A$10*D4-(D4+D4A2*$A$10)/$A$4)/
4
15 a(Y -) =
(D4*$A$10+(D4+D4A2*$A$10)/$A$8)
16
0.30 F4 = E4*$A$4*$A$6/$A3.8
17 [NH3] =
18
0.1
19 ßi =
ß2 =
p3 =
ß4 =
20 1.51E+02 2.69E+04 5.50E+06 5.01 E+08
(b)
10
15
20
Volume of EDTA (mL)
25
30
35
EDTA Titrations
A
1
B
O
D
F
E
Titration of 5 0 mL of 0.05 M N i z + w i t h 0.1 M EDTA/pH 1 1 / 0 . 1 M Oxalate
2
3
v„ =
50
6
7
=
CHXTA
a
9
6.97
0.005
4
5
0.01
Kf"4.42E+13
10
M
pM
CM =
1.07E-07
[Mit«
•
VEDTA
4.94E-03
0.008
0.210
1.342
7.00
1.00E-07
4.61E-03
0.054
7.20
6.31 E-08
2.91 E-03
0.324
8.106
7.50
3.16E-08
1.46E-03
0.618
15.461
6.00
1.00E-08
4 . 6 1 E-04
0.868
21.696
23.649
8.40
3.98E-09
1.83E-04
0.946
8.80
1.58E-09
7.30E-05
0.978
24.456
0.996
24.891
11 u(Ni ') =
2.17E-05
12
9.50
3.16E-10
1.46E-05
10.50
3.16E-11
1.46E-06
1.000
24.989
13 l o g K f «
12.80
1.58E-13
7.30E-09
1.000
25.000
14.00
1.00E-14
4 . 6 1 E-10
1.000
25.001
15.00
1.00E-15
4.61E-11
1.000
25.012
16.00
1.00E-16
4.61E-12
1.005
25.123
26.229
2
18.4
14
15 a(Y«-) =
0.81
16
2
17 [ O x a l a t e ] =
0.1
18
19 Pi =
20
1.45E+05
21 \h =
3.16E+06
22
17.00
1.00E-17
4.61E-13
1.049
17.40
3.98E-18
1.63E-13
1.123
28.086
17.60
2.51 E-18
1.16E-13
1.196
29.892
17.80
1.58E-18
7.30E-14
1.310
32.753
17.90
1.26E-18
5.80 E-14
1.390
34.760
18.00
1.00E-18
4 . 6 1 E-14
1.491
37.287
23
A
24 A 1 0 = A 1 6 * A 1 2 * 1 0 A 1 4
A
25 A 1 2 = 1 / ( 1 + A 2 0 * A 1 8 + A 2 2 * A 1 8 2 )
A
26 C4 = 1 0 - B 4
27
28
29
30
11-21.
D4 = C 4 / $ A $ 1 2
E4 = ( 1 + $ A $ 1 0 * D 4 - ( D 4 + D 4 * D 4 * $ A $ 1 0 ) / $ A $ 4 ) /
| ( D 4 * $ A $ 1 0 + ( D 4 + D 4 * D 4 * $ A $ 1 0 ) / $ A !( 8 )
F4 = E 4 * $ A $ 4 * $ A $ 6 / $ A $ 8
[L] + [ML] + 2[ML2] CMVM
[L] + aML vM+VL
y^yl
,
+ 2otML
CMVM
2 VM+VL -
CLVL
KM+KL
Multiply both sides by KM + KL:
[L] KM + [L] KL + (XMLCM VM + 2aML2CM ^M - CLKL
Collect terms
yL
TM=
KL ([L] - CL) - KM (-[L] - a M LC M - 2aML2CM)
[L] + aML^M + 2aML2^M
CWL]
138
Chapter 11
Divide the denominator by CL and divide the numerator by CM to obtainfy,the
fraction of the way to the equivalence point:
<j> -
ctML + 2 a M L
2
LU
C M KM
0.06
11-22.
CM +
CLKL
1 - CL
-i—i
i
i
i
S 0.03
c 0.02
1
2
Volume of L added
A
B
C
D
E
1 Copper-acetate compte)<es ML and ML?
2
3 CM =
4
0,05
5 VM =
6
10
7 C(ligand)
8
0.5
PL
[L]
4.0 0.0001
3.0 0.0010
2.8
2.6
2.4
2.2
0.0016
0.0025
0.0040
0.00G3
0.983
0.852
a«
0.017
0.145
0.781
0.688
0.573
0.210
0.294
0.388
«M
F
G
H
1
<XML2
4
V(ligand)
[M]
J
K
[ML]
[ML2]
0.ÛÛO
0.004
0.019
0.172
0.019 0.0491
0.172 0.0419
0.0008 0.0000
0.0071 0.0002
0.008
0.019
0.039
0.076
0.260
0.383
0.550
0.766
0.260
0.383
0.550
0.766
0.0103
0.0141
0.0184
0.0222
1.039
1.199
1.039 0.0145
1.199 0.0117
0.0246 0.0062
0.0250 0.0080
1.377
1.5791
1.808
2.073
2.385
2.761
2.981
1.377
1.579
1.808
2.073
2.385
2.761
2.981
0.0248
0.0240
0.0226
0.0209
0.0187
0.0164
0.0152
0.4461 0.478
9 ßi =
10
2.0 0.0100
170
0.319
0.543
0.137
0.262
0.560
0.178
1.9 0.0126
11 ß 2 =
12
4300
1.8 0.0158
0.209
0.564
0.226
13
1.7 0.0200
0.280
0.164
0.556
14
1.6 0.0251
0.125
0.535
0.340
15
1.5 0.0316
0.094
0.504
0.403
16
1.4 0.0398
0.467
0.0691 0.464
17
1.3 0.0501
0.532
0.049!
0.419
18
1.3 0.0562
0.563
0.0411 0.396
19
20 C4 = 10*-B4
21 D4 = 1/(1+$A$10*C4+$/ i$12*C4*C4)
22 E4 = $A$10*C4/(1+SA$10*C4+$A$12*C4*( Ml
23 F4 = $A$12'C4*C4/(1+$ A$10'C4+$A$12*CM*C4)
24 G4 = (C4/$A$4+E4+2*F<l)/(1-C4/$A$8)
0.0381
0.0331
0.0272
0.0207
0.0092
0.0071
0.0053
0.0039
0.0028
0.0019
0.0016
H4 = G4*$A&4*$A$6/$AS8
I4 ^ D4*$A$4'$A$6/($A$6+H4)
J4 = E4',$A$4*$A$6/($A$6^-H4)
K4 = F4*SA$4*$A$6/($A$6-(-H4)
1
0.0004
0.0009
0.0019
0.0035
0.0099
0.0121
0.0144
0.0167
0.0189
0.0208
0.0217
139
EDTA Titrations
11-23.
Only a small amount of indicator is employed. Most of the Mg2+ is not bound to
the indicator. Free Mg 2+ reacts with EDTA before Mgln reacts. Therefore, the
concentration of Mgln is constant until all of Mg 2+ has been consumed. Only
when Mgln begins to react does the color change.
11-24.
1.
2.
3.
4.
11-25.
Hin 2- , wine red, blue
11-26.
Buffer (a) (pH 6-7) will give a yellow -»- blue color change that will be easier to
observe than the violet -+ blue change expected with the other buffers.
11-27.
A back titration is necessary if the analyte precipitates in the absence of EDTA, if
With metal ion indicators
With a mercury electrode
With an ion-selective electrode
With a glass electrode
it reacts too slowly with EDTA, or if it blocks the indicator.
11-28.
In a displacement titration, analyte displaces a metal ion from a complex. The
displaced metal ion is then titrated. An example is the liberation of Ni 2+ from
Ni(CN)J" by the analyte Ag+. The liberated Ni2< is then titrated by EDTA to find
out how much Ag + was present.
11-29.
The Mg 2+ in a solution of Mg 2+ and Fe 3+ can be titrated by EDTA if the Fe 3+ is
masked with CN" to form Fe(CN)J", which does not react with EDTA,
11-30.
Hardness refers to the total concentration of alkaline earth cations in water, which
normally means [Ca2+] + [Mg 2+ ]. Hardness gets its name from the reaction of
these cations with soap to form insoluble curds. Temporary hardness, due to
Ca(HC03)2, is lost by precipitation of CaC03(.s) upon heating. Permanent
hardness derived from other salts, such as CaS04, ¡s not affected by heat.
11-31.
(50.0 mL)(0.0100 mmol/mL) - 0.500 mmol Ca 2+ , which requires 0.500 mmol
EDTA = 10.0 mL EDTA.
0.500 mmol Al 3+ requires the same amount of EDTA, 10.0 mL.
11-32.
mmol EDTA = mmol Ni 2+ + mmol Zn 2+
1.250 =
x
+ 0.250 => 1.000 mmol Ni 2+ in 50.0 mL = 0.020 0 M
140
11-33.
Chapter 11
The formula mass of MgS0 4 is 120.37. The 50.0 mL aliquot contains
(50.0 mL^ ( 0.450 g S
L500mLjll20.37g/molJ = 0.373 8 mmol of Mg2+
37.6 mL of EDTA reacts with this much Mg 2+ , so the EDTA solution contains
0.373 8 mmol / 37.6 mL = 9.943 x lO"3 mmol/mL. The formula mass of CaC0 3
is 100.09. 1.00 mL of EDTA will react with 9.943 x 10-3 mmol of CaC0 3 =
0.995 mg.
11-34.
30.10 mL Ni 2+ reacted with 39.35 mL 0.013 07 M EDTA, so Ni 2+ molarity is
rxr? +1
^
J
=
(39.35 mLXO.013 07 mol/L)
30ÏÏÏÏ5L
=
nn
M
° 0 1 7 0 9 M-
25.00 mL Ni 2+ contains 0.427 2 mmol Ni 2+ . 10.15 mL EDTA = 0.132 7 mmol
EDTA. The Ni 24 which must have reacted with CN" was 0.427 2 - 0.132 7 =
0.294 5 mmol. Cyanide reacting with Ni 2+ must have been (4)(0.294 5 mmol) =
1.178 mmol. Original [CN-] = 1.178 mmol/12.73 mL = 0.092 54 M.
11-35.
For 1.00 mL of unknown:
25.00 mL of EDTA = 0.9680 mmol
23.54 mL of Zn2+
Co
2+
+ Ni
2+
= 0.500 7 mmol
= 0.4673 mmol
For 2.000 mL of unknown:
25.00 mL of EDTA = 0.968 0 mmol
25.63 mL of Zn2^
Ni
2+
in 2.000 mL
= 0.545 2 mmol
= 0.422 8 mmol
Co2+ in 2.000 mL of unknown = 2 (0.4673) - 0.422 8 = 0.511 8 mmol. The Co2+
will react with 0.511 8 mmol of EDTA, leaving 0.968 0 - 0.511 8 = 0.456 2 mmol
i ivTA
i -»
0.456 2 mmol
EDTA.
mL Zn needed = 0 .02127 mmol/mL = 2 1 A 5 m L
11-36.
Total EDTA
Mg 2+ required
Ni 2+ + Zn 2+
= (25.0 mL) (0.045 2 M)
=
1.130 mmol
(12.4 mL) (0.012 3 M) =
0.153 mmol
= 0.977 mmol
Zn 2+ = EDTA displaced by 2,3-dimercapto-l-propanol
141
EDTA Titrations
= (29.2 mL) (0.0123 M) = 0.359 mmol
=> Ni 2+ = 0.977 - 0.359 = 0.618 mmol; [Ni2+] =
[ ^ ] = ^ r = 0 . 0 0 7
11-37.
1 8
5Q Q m L
=0.0124 M
M
The precipitation reaction is Cu + + S " —* CuS (s).
Total Cu2+ used = (25.00 mL) (0.043 32 M) = 1.083 0 mmol
" Excess Cu2+
mmol of S2"
- (12.11 mL) (0.039 27 M) = 0.475 6 mmol
= 0.6074 mmol
[S 2 ] = 0.607 4 mmol/25.00 mL = 0.024 30 M
11-38.
mmol Bi in reaction - (25.00 mL) (0.08640 M) = 2.160 mmol
EDTA required = (14.24 mL) (0.043 7 M) = 0.622 mmol
mmol Bi that reacted with Cs = 2.160 - 0.622 = 1.538 mmol
Since 2 mol Bi react with 3 mol Cs to give CS3BÍ2I9,
3
mmol Cs + in unknown = 2 (1-538) = 2.307 mmol
wi-lg 1 -^»*
11-39.
Total standard Ba2+ + Zn2+ added to the sulfate was (5.000 mL)(0.014 63 M
BaCl2) + (1.000 mL)(0.01000 M ZnCl2) - 0.083 15 mmol. Total EDTA
required was (2.39 mL)(0.009 63 M) = 0.023 02 mmol. Therefore, the original
solid must have contained 0.083 15 - 0.023 02 = 0.060 I3 mmol sulfur (which
made 0.060 I3 mmol sulfate that precipitated 0.060 13 mmol Ba2+). The mass of
sulfur was (0.060 I3 mmol)(32.066 mg/mmol) - 1.92g mg. wr% S = 100 x
(1.928 mg S/5.89 mg sphalerite) = 32.7 wt%. Theoretical wt% S in pure ZnS =
100*(32.066gS/97.46gZnS) = 32.90 wt%.
CHAPTER 12
ADVANCED TOPICS IN EQUILIBRIUM
12-1.
As pH is lowered, [H+] increases. H + reacts with basic anions to increase the
solubility of their salts. Dissolution of minerals such as galena and cerussitc
increases the concentration of Pb 2+ in the environment.
12-2.
Galena:
?bS(s) + H+ ^
Pb 2+ + HS-
Cerussitc:
PbC0 3 (s) + H + ^
Pb2* + HCOj
(a) Hydroxybenzcne = HA with pÄHA = 9.997
Mixture contains 0.010 0 mol HA and 0.005 0 mol KOH in 1.00 L.
Chemical reactions:
HA ^
A" + H+
H 2 0 ^ H+ + OIP
KUA = ^
^
= 10-9.997
Kw = [Hf][OH-] = lO-'4-«»
Charge balance:
[H+] + [K+] = [OH-] + [A-]
Mass balances:
[K+] = 0.005 0 M
[HA] + [A-] = 0.0100 M = F A
We have 5 equations and 5 chemical species.
Fractional composition equations:
[H+]FA
;
[HA]
= CCHAFA
[A"] = a A - F A =
[H+l + A-HA
KUAFA
[H+] + KHA
Substitute concentration expressions into the charge balance:
[H+] + [0.005 0] = AV[H + ] + a A - F A
(A)
We could solve Equation A for [H+] by using the solution to a quadratic
equation. Instead, we will use Solver in the following spreadsheet, with an
initial guess of pH =10 in cell H9. Select Solver and choose Options. Set
Precision to Ic-16 and click OK. In the Solver window, Set Target Cell FJ2
Equal To Value of 0 By Changing Cells H9. Click Solve and Solver finds pH
= 9.98 in cell H9, giving a net charge near 0 in cell F12.
142
143
Advanced Topics in Equilibrium
6
7
8
9
10
11
12
13
14
15
16
17
Species in charge balance:
[A] =
[H+] =
1.05E-10
[OHl =
5.00E-03
Kl =
4.90E-03
9.56E-05
H
G
D
E
F
A l
B
|C
Mixture
of
0.010
M
HA
and
0.005
M
NaOH
1
2
[K>
0.005
0.010
3 FA =
14.000
pK,, =
9.997
4 PKHA =
1.00E-14
Kw =
1.01 E-10|
5 KHA =
I
Other concentrations:
[HA]=
I 5.10E-03
pH =
9980
T initial value is a guess
I
Positive charge minus negative charge -3.00E-18 = B8+B9-E8-E9
Formulas:
B8= 10MH9
B5 = 10*-B4
A
B9-E3
E5=10 -E4
E9
= E5/B8
E8 = B5*B3/(B8+B5)
H8 = B8*B3/(B8+B5)
I
(b) In Chapter 8, we would have said that there is enough KOH to neutralize half
of the HA. Therefore, [HA] = [A"].
pH - p*a + log([A"]/[HA]) = pKa + log(l) = pKa = 10.00. The
systematic treatment of equilibrium in the spreadsheet gave pH = 9.98.
(c) If we dilute HA to 0.000 10 M and KOH to 0.000 50 in cells B3 and E3, then
Solver finds a pH of 9.45. It makes sense that as the solution becomes more
dilute, the pH must move toward 7.
12-3.
We use effective equilibrium constants, K', defined as follows:
*HA =
[H+]YH+[A-]YA[HA]YHA
Ku;
LLULA-j
" " *
[HA]
= *HA
YHA
YH+YA-
Kw = [H + rftHOH-]yoH- = 10->3-995
Kw -
Ku
+
YH YOH-
= [H + ][OH-]
[OH"] = 0 [ H + ]
pH = -log([H+]YH+)
The following spreadsheet shows the beginning of the first iteration with an initial
ionic strength of 0 in cell CI 7. Execute Solver to find the pH in cell H13 that
produces a net charge of 0 in cell El6. Solver finds pH = 9.98 and ionic strength =>
0.005 0 in cell CI8. Write 0.0050 in cell C17 and execute Solver again to find
144
Chapter 12
pH = 9.95 and ionic strength - 0.0050 in cell C18. Since the ionic strength did not
change after the 2 nd iteration, we are finished. The pH is 9.95.
A
1 Mixture of 0.010
¿
3 FA =
4 PK»A =
5
KHA
6
7
8
9
10
11
12
13
14
15
=
B
C
D
E
I F
G
M HA and 0.005 M NaOH with activity coefficients
|
[K*] =
0.010
0.005
9.997
pK* =
13.995
1.01 E-10
K* =
1.01E-14
Activity coefficients:
H* =
1.00
OH" =
1.00
A
HA
Species in charge balance:
[HJ=
1.00E-10
[K] =
0.005
[A] =
[OHT =
H
1.00
1.00
5.02E-03
101E-04
Other concentrations:
[HA] =
4.98E-03
pH =
10.000
t initial value is a guess
|
|
-1.18E-04 = B12+B13-E12-E13
1b Positive charge minus negative charge =
17 Ionic strength =
0.0000 <- initial va uelsO
I
I
18 New ionic strength = I 0.0051 «- substitut e this value into cell C17 for next iteration
19
20 Formulas:
21 B5 = (10A-E¡4)*E9/(B8*E8)
E9 = 1
B13 = E3
22 E5 = (10A^E4)/(B8*B9) ¡
23 B8 = B9 = E8 = 10A(-0.51*1A2*(SQRT($C$1 ry(i+sQRi"($C$17))-0.3*$C$17))
24 B12 = (10A- H13)/B8
2b E12 = B5'B 3/(B12+B5))
E13 = E5/B12
H12 = B12*B3/(B12+B5)
2b C18 = 0.5*( B12+B13+E12+E13)
12-4.
Abbreviating the protonated form of glycine as H2G + , we write
H2G+ ^
HG
HG + H +
K\ =
G" + H+
K2 =
[HG]YHC[H+]YH+
pA'i - 2.350
[H2G+]YH2G+
[G-]YG-[H + ]YH+
pK2 = 9.778
[HG]YHG
At u = 0.1 M, the activity coefficient of a monovalent ion is 0.78. The activity
coefficient of a neutral molecule is I. Putting these coefficients into the
expressions for K\ and K2 gives
«»W W *
K
2' ^HGT^^
0 1
1
M
M) = H
Ä . u^S
O^
(1X0.78)
YHGYH+
YHG
1
) = « 2 ^ = I»"9778 (0.78X0.78)
m I0.2350
= 10-9.562
I
145
Advanced Topics in Equilibrium
The predicted values arc pA'i = 2.350 and pK2 = 9.562. Values from fitting the
data in the spreadsheet arc 2.312 and 9.625. The change from pA'i to pA'i is
expected to be zero and it is observed to be -0.038. The change from pK2 to pAT2
is expected to be -0.216 and it is observed to be -0.153.
12-5.
Ethylcncdiamine = B from diprotic H 2 B 2+
pA'i - 6.848
pK2 - 9.928
Mixture contains 0.100 mol B and 0.035 mol HBr in 1.00 L.
Chemical reactions:
H 2 B 2+ ^ HB+ + H +
HB + ^ B + H+
A-| = 10- 6 8 4 8
K2 = 10-9 928
H20 ^
Kw = 10-14.00
H+ + OH-
Charge balance:
[H+] + 2[H 2 B 2+ ] + [HB+] = [OH"] + [Br]
Mass balances:
[Br] = 0.035 M;
[H2B+] + [HB] + [B] = 0.100 M = F B
We have 6 equations and 6 chemical species, so there is enough information.
Fractional composition equations:
[H + ] 2 F B
2+
[H 2 B ] - ctH2B2+ FB - [ H + ] 2 + [H+]K] +
K]K2
KX[H+]FB
[HB ] - aiiB+Fe - [H+] + [H+]Ki+KiK2
4
[B] - a B F B
2
K\K2FB
== [H+] + [H+1A-! + A-iA-2
2
Substitute into charge balance:
[H+] + 2(XH2B2+ F B + a H B + F B = KJ[H+] + [0.035 M]
(A)
We solve Equation A for [H+] by using Solver in the following spreadsheet, with
an initial guess of pH = 10 in cell HI 1. Select Solver and choose Options. Set
Precision to 1E-16 and click OK. In the Solver window, Set Target Cell E14
Equal To Value of 0 By Changing Cells HI 1. Click Solve and Solver finds pH =
10.194 in cell H11, giving a net charge of ~10"17 in cell E14.
Chapter 12
I C I
D
A
B
1 Mixture of 0.100 M B and 0.035 M HBr
2
3 FB =
|[Br-) =
0.100
4 pKi =
6.848
pK* =
5 pK2 =
6 K,=
9.928
Kw
=
E
F
G
H
0.035
14.000
1.00E-14
1.42E-07
7 K2 =
1.18E-10
8
y Concentrations:
10 [H I =
I 6.39E-11
[HZB2*] =
1.58E-05
[B] =
6.49E-02
11 (Br) =
3.50E-02
[HB*] =
3.51 E-02
pH=
10.194
12
13
14
15
16
17
18
19
20
1.56F-04
[Orf] =
I
T initial value is a quess
Positive ch arge minus negative charge -1.70E-17
Formulas: j
B6 = 10A-B4
B7 = 10A-B5
E5 = 10A-E4
A
B10= 10 -H11
B11 = E3
B12 = E5/B10
A
E10 = B10 2*$B$3/(B10A2+B10*$BS6+$B$6*$B$7]
b l l = B1O'$B$6*SB$3/(B10A2+B10*$B$6+$B$6*$B$7)
H10 = 5BSt rsBsrsBb 3/(B1 0A2+B10*$B$6+SBS6*$B$7)
In your earlier life, you would have solved this problem by noting that 0.035 mol
HBr converts 0.035 mol B into 0.035 mol HB + , leaving (0.100 - 0.035) mol B.
pH - pK2 + log [ H B + j = 9.928 + log Q^Q35 = 10.197 (close to spreadsheet answer)
12-6.
Benzene-1,2,3-tricarboxylic acid = H3A with pA'i - 2.86, ptf2 = 4.30, pÂ/j = 6.28;
Imidazole = HB from diprotic H 2 B + with pA'i = 6.993, pK2 » 14.5
Mixture contains 0.040 mol H3A, 0.030 mol HB, and 0.035 mol NaOH in 1.00 L.
Charge balance:
[H+] + [H2B+] + [Na+] = [OH"] + [H2A~] + 2[HA 2 '] + 3[A 3 '] + [B"]
Substitute fractional composition equations into charge balance:
[H+] + an 2 B + FB +[0.035]
= AV[H + ] + aH2A- FA + 2ctH2A2- FA + 3CIHA3- FA + aß- F B
We solve for [H+] with the following spreadsheet, with an initial guess of pH = 7
in cell H14. Select Solver and choose Options. Set Precision to le-16 and click
OK. In Solver, Set Target Cell FJ6 Equal To Value of 0 By Changing Cells HU.
Click Solve and Solver finds pH = 4.52 in cell H14, giving a net charge of ~10" 16
in cell E16.
147
Advanced Topics in Equilibrium
HL
I
I B ZI
Mixture of 0.040 M H A 0,030 M HB. and 0.035 M NaOH
0.040
2BÖ
FA =
pK,=
pK2 =
pK3 =
L
K,=
K2 =
FB =
=
PKHîB
4.30
PKHB
=
6.28
KH2B
"
1.4E-03
KHB
=
0.030
[Na]
H
6,993
pK-
0.035
14.000
14.5
K» =
1.00E-14
1.02E-07
3.28E-10
0.035
3.16E-15
5-0E-05
5.2E-07
K3 =
10
11 Concentrations
_3X)5E-05
12 [H*)=
3.27E-04
13 [H3A] =
14 [H a A] =
15 [HA'l =
[A' I =
[HZB*] =
421E-04
2.99E-02
[0H1 =
[Na1 =
1.48E-02
[HB] =
9.98E-05
2.44E-02
[B] =
1.04E-14
4.516
pH =
T initial value is a guess
16 Positive charge minus negative charge j -2.42E-17J
17
18 Formulas:
B9= 10A-B6
B8= ICr^-Bö
B7 - 10A-B4
E7= lO^ES
20 Ê6 = 10*-E4
H12 = H5/B12
H13=H3
21 B12= 10MJ14
A
A
A
22 B13=$B$12 3*$B$3/($B$12 3+$B$12 2*$B$7+$B$12*$B$7*$B$8+$8$7*$B$8*$B$9)
A
A
A
23 B14=$B$12 2*$B$7*$B$3/{$B$12 3+$B$12 2*$B$7+$B$12*$B$7*$B$8+$B$7*$B$8*$B$9)
A
A
24 B15=$B$12*$B$7*$B$8*$B$3/($B$12 3+$B$12 2*$B$7+$B$12*$B$7*$B$8+$B$7*$B$8*$B$
A
A
25 E12= B$7*B$8*B$9*B$3/(B$12 3+B$12 2*B$7+B$12*B$7*B$8+BS7*B$8*BS9)
A
A
26 E13 = $B$12 2*$E$3/($B$12 2+$B$12'$E$6+$E$6'$E$7)
A
27 E14 = $B$12*$E$6*$E$3/($B$12 2+$B$12*$E$6+$E$6*$E$7)
,
A
,
28 E15 = $E$6*$E$7 $E$3/($B$12 2+$B$12'$E$6t-$E$6 SE$7)
12-7.
Arginine - HA from H 3 A 2+ with pA'i = 1.823, pA2 - 8.991, pK3 - 1 2 . 1 ; glutamic
acid = H2G from H 3 G + with pA'i = 2.160, pK2 = 4.30, pA:3 = 9.96
Mixture contains 0.020 mol arginine, 0.030 mol glutamic acid, and 0.005 mol
KOH in 1.00 L. F A = 0.020 M and F G = 0.030 M.
Charge balance:
[H+] + 2[H 3 A 2+ ] + [H 2 A + ] + [H3G+] + [K+] = [OH"] + [A"] + [HG'] + 2[G 2 ']
Substitute fractional composition equations into charge balance:
[H+] + 2otH3A2+ F A + <XH2A+ F A + aH3G+ F G + [0.005]
= AV[H + ] + aA- F A + ctHG- FG + 2oG2- F G
148
1
Chapter 12
A
B
C | D
E l F l G l H
Mixture of 0.020 M arginine, 0.030 M glutamic acid, and 0.005 M KOH
2
3 FA =
4 pK,=
0.020
5
PKî =
8.991
6 PK3 =
12.1
7 K,=
1.5E-02
8 K2 =
1.0E-09
0.030
[K>
0.005
P^HSO
3
2.160
pK.=
14.00
PKHKî
=
4.30
Kw=
1.00E-14
FB =
1.823
I
=
9.96
K,H3G=
6.9E-03
KH20 =
5.0E-05
KH6=
1.1E-10
PKHO
9 K3 =
7.9E-13
10
11 Concentrations
12 [HjA ] =
1.32E-05
[HjGl=
7.14E-06
(H*) =
9.94E-06
13
2.00E-02
[HjG] =
4.96E-03
[OH"]=
1.01E-09
14 [HA] =
2.05E-06
|HG ] =
2.50E-02
[K>
0.005
15 (Al =
1-64E-13
[G2 ] =
2.76E-07
pH =
5.003
[HA] =
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
J
î initial value is a guess
Positive charge minus negative charge
-9.94E-17J
= 2'B12+B 13+E12+H12+H14-B15-E1 4-2*E15-H13
Formulas:
B7=10 A -B4
E7 = 10A-E4
H12=10 A -H15
B9 = 10A-B6
E9 = 10A-E6
H14 = H3
D \¿ = 3>M3)1¿"J ¡MüfcOrT;» H5)1^3+$H$12A2*$B$7+SHS12*SB$7*$B$8+$BS7*$B$8*$B$9>
B13=$H$12 A 2*$B$7'$B$3/($H$12 A 3+$H$12 A 2*$B$7+$H$12*$B$7*$B$8+$B$7*$B$8*$B$9)
B14=$H$12*$B$7*$B$8*$B$3/($H$12 A 3+$H$12 A 2*$B$7+$H$12*$B$7*$B$8+$B$7*$B$8*$B 59)
B15=$B$7*$B$8*$B$9*$B$3/($H$12 A 3+$H$12 A 2*$B$7+$H$12*$B$7 , $B$8+$B$7*$B$8*$B$£ >)
E12=$H$12 A 3"$E$3/($H$12 A 3+$H$12 A 2*$E$7+$H$12 A $E$7*$E$8+$E$7*$E$8*$E$9)
E13=$H$12 A 2*$E$7*$E$3/($H$12 A 3+$H$12 A 2 É $E$7+$H$12 A $E$7'$E$8+$E$7*$E$8"$E$9)
E14=$H$12*$E$7*$E$8*$E$3/($H$12 A 3+$H$12 A 2*$E$7+$H$12'$E$7*$E$8+$E$7*ÎE$8-$E$9)
E15=$E$7*$E$8*$E$9*$E$3/($H$12 A 3+$H$12 A 2*$E$7+$HS12'$E$7*$E$8+$E$7*SE$8*$E$9)
B8= If^-BS
E8=10 A -E5
H13 = H5/H12
We solve for [H+] with the spreadsheet, with an initial guess of pH = 3 in cell H15.
Select Solver and choose Options. Set Precision to lc-16 and click OK, In Solver,
Set Target Cell E17 Equal To Value of 0 By Changing Cells H55. Click Solve and
Solver finds pH = 5.00 in cell H15, giving a net charge of-lO' 1 6 in cell E17.
12-8.
H 3 A 2+ ^
H2A+ ^
HA ^
H2A+ + H+
HA + H +
A" + H +
AT,
A2
A3
[H2A + ] Y H 2 A^[H + ]YH^
[H3A 2i ]y„ 3A 2 +
[HA]YHA[H + ]YM
[H2A + ]YH 2 A+
= j o-l.823
= 10-8-991
[A"]yA-[H+)YH^
= lO"'2 I
[HA]yHA
149
Advanced Topics in Equilibrium
f
K\ = ZT,
A3 - Ki
H3G
+
[H2A+][H+]
YH3A2+ '
2+
[H 3 A ]
JHJA+YH+J
^YHA }
V.YA-YHV "
^H3G
KH G
2
HG" T* G2" + H+
^H3G
^HG
==
IjHAW
[H2G]YH2G[H+]YII>
H G- + H+
(
7II,\
[HA1ÍH+]
[H2A+]
\A1\W]
lIIAl
H2G + H+
H2G
K = K:
KHG
7H3G+ >
[H2GKIP]
[H3G+]
^ G YH GYH«J
2
YHG-
^IIG I YG2-YH+;
V
=
= ! 0-2.160
[H 3 G + ]YH 3 G+
[HG]YHG-[H+JYH^ = 10-4.30
[H2G]YH2G
[G 2 "]YG2-[H + ]YH
= 10-9.96
[HG-]ynG' YH2G
*H2G » «HjG^
[H2G]
[G£][H!1
[HG"]
In the next spreadsheet, [H ] in cell H18 is computed from (10-PHVyn+- Don't
forget that activity coefficient! Ionic strength in cell D24 was initially set to 0, and
activity coefficients are computed in cells A13:H15. From the activity
coefficients, effective equilibrium constants are computed in cells I14:H10. pH =
3 is guessed in cell H21. From pH and the À" values, concentrations are computed
in cells A18:H21. Solver is used to vary pH in cell H21 until the net charge in cell
H23 is 0. If Solver does not find an answer, try a different initial value for pH or
increase Precision in the Options window of Solver. A value of le-16 generally
works, but for some problems 1 need larger numbers, such as 1E-10. The ionic
strength computed in cell D25 is then entered into cell D24 and Solver is executed
again. The process is complete when ionic strength no longer changes, pi I
computed with activities is 4.95 and p. - 0.025 M. When we found pH without
activities in the previous problem, the pH was 5.00.
+
Iteration
ionic strength
pH
1
2
0
0.025 0
0.025 1
5.003
4.939
4.939
3
150
Chapter 12
A
B
C
D
F
| F
G
1 Mixture of 0.020 M arginine, 0.030 M glutamic acid, and 0.005 M KOH
2 Solved with Davies activity coefficients
3 FA =
0.020
0.030
FB =
[K] =
4 pK,=
1.823
2.160
PKH3G =
K,'=
5 pK 2 =
PKHJQ =
8.991
4.30
K 2 "=
6 pK 3 =
12.1
PKHG =
7 K,=
1.5E-02
8 Kz =
1.0E-09
9 K3 =
7.9E-13
KHG
10 pK w =
13.995
9.96
1.1 E-02
1.0E-09
1.1 E-12
KH3G
6.9E-03
IKHSG'S
6.9E-03
KH2G
=
5.0E-05
I W =
6.8E-05
=
Kw =
1.1E-10
1.01E-14
KHG'
=
2.01E-10J
Kw' =
1.37E-14
0.86
H' =
0.86
0.54
OH' =
H 3 G* =
0.86
+
14 H?A =
HG" =
G* =
I
0.005
=
11
12 Davies activity coefficient
13 H 3 A ¿ *=
0.54
0.86
K 3 '=
H
15 A =
0.86
16
17 Concentrations
18 [HjA j ! *]=
2.41 E-05
[H3G*] =
19 [HzA+] =
2.00E-02
[H 2 G] =
9.58E-06J
4.95E-031
[H*] =
[OH1 =
20 [HA] =
21 [ A ] =
1.52E-06
[HG ] =
2.50E-02
[IC] =
0.005
1.22E-13
[G"] =
3.76E-07
pH =
4.939|
0.86
^^—
1.34E-05
1.02E-09
22
î initial value is a guess
23 Positive charge minus negativ e charge = -8.86E-18
7A
Ionic strength =
0.0251 <- initial valí e is 0
2b
New ionic strength =
0.0251 «- substitute this value into cell D24
26
for next interation
27 Formulas
28 K,' = B7*B13/(B14*H14)
K 2 ' = B 8 * B 1 4/{H14)
K : J '=B9/(B15*H14)
29 K H 3 B' = E7*E13/H14
IW=E8/(E14*H14)
KHB' = E 9 * E 1 4 / ( E 1 5 * H 1 4 )
a
30 Kw -«E10/(H14*H15)
[H+] = {10 A -H21)/H14
[OH-] = H10/H18
31 Activity coefficient = 10^-0.51*1:harge A 2*(SQRT($D$24V(1-i SQRT($D$24))-0.3*$D$24))
A
A
32 Denoml =($H$18 3+$H$18 2 *$H$4+$H$ 18*SH$4*SH$5+$H$4*$H$5*$H$6)
33 [H3A' ] = $H$18 A 3*$B$3/Denom1
[HjA*] = $H$18 A 2*$H$4*$B$3/Denom1
34 [HA] = SH$J 8*$H$4*$H$5*$B$3/Denom1
[AT = $H$4*$H$5*$H$6*$B$3/Denom1
35 Denom2 = ( $H$ 18 A 3+$HS18 A 2*$H$7+$H! 18*$H$7*$H$8+$H$7*$H$8*$H$9)
36 [H 3 G + ] = $H$18 A 3*$E$3/Denom2
[H 2 G] = $H$18 A 2*$H$7*$E$3/Denom2
37 [HG1 = $H$18*$H$7*$H$8*$E$3/Denom2
|[G2T = $H$7*$H$8*$H$9*SE$3/Denom2
#
38 E23 = 2 B18+B19+E18+H18+H20-B21-E20-2*E21-H19
39 D25= 0.5*{4*B18+B19+B21+E18+E20+4*E21+H18+H19+H20)
12-9.
(a) This is the same problem that was worked in Section 12-2, but with [KH2PO4]
= 0.008 695 m and [Na2HP04] = 0.030 43 m in cells B3 and B4 of the
spreadsheet in Figure 12-4. Beginning with ionic strength = 0 in cell C19 and
pH = 7 in cell H15, wc find the value of pHrequiredto make the net charge 0
151
Advanced Topics in Equilibrium
in cell El 8 by using Solver with Precision = I e-16. The first iteration gives pH
= 7.742. The ionic strength in cell C20 after the first (and all subsequent)
iterations is 0.100 m. Typing this value in cell CI9 and performing a second
iteration gives pH = 7.420.
(b) To use the Debye-Hückel equation, we can compute activity coefficients with
ion size parameters from Table 7-1 or wc can just use activity coefficients from
Table 7-1 for p. = 0.1 M:
A
B
C
|
D
8 Activity coefficients from table iin textbook:
0.83
H3P =
9 H* =
10 OH =
0.76
H2P' =
E
F
G
1.00 (fixed at 1) HP2
=
0.78
H
P^ =
0.36
0.10
These activity coefficients produce a pi I of 7.403 after executing Solver to
reduce the net charge to 0 in cell El8.
12-10.
EDTA = H4A from hexaprotic H 6 A 2+ with pA'i = 0.0, pA2 = 1.5, pK3 = 2.00, pA^
= 2.69, pK$ = 6.13, pAö = 10.37; Lysine - HL from triprotic H 3 L 2+ with pAi =
1 . 7 7 ^ 2 = 9.07^*3=10.82
Mixture contains 0.040 mol H4A, 0.030 mol HL, and 0.050 mol NaOH in 1.00 L.
Charge balance:
[H+] + 2[H 6 A 2+ ] + [H5A ' ] + 2[H 3 L 2+ ] + [H2L+] + [Na+]
= [OH"] + [H3A-] + 2[H2A2"] + 3[HA3-] + 4[A*"] + [LI
Substitute fractional composition equations into charge balance:
[H+] + 2<xH6A2+ FA + aH5A+ F A + 2aH3L2+ F L + aH2L+ F L + [0.050]
= AV[H + ] + a H3 A- FA + 2an2A2- FA + 3aHA3- FA + 4a A i- F A + a L - F L
Wc solve this equation for pH with the following spreadsheet, with an initial guess
of pll = 7 in cell H18. In Solver, Set Target Cell EJ9 Equal To Value of 0 By
Changing Cells m 8 . Click OK and Solver finds pH = 4.44 in cell H18.
152
Chapter 12
A
B
C
D
E
| F |
1 Mixture of 0.040 M H+A. 0.030 M HL, and 0.05 M NaOH
i
2
3
FA =
4
pK,=
5
6
0.040
FL =
0.030
0.0
PKL,=
1.77
pK 2 =
1.5
PKL2 =
9.07
pKs =
2.0
7 PK4 =
2.69
KLI
8
pKs =
6.13
K|_2 _
9
pKe -
10.37
|KU =
PKL3 =
=
10.82
1.70E-02
G
H
[
[Na1 =
0050
pK* =
K„ -
14.00
1.00E-14
8.51E-10r
1.51E-11
10 K , =
1.00E+00
K3 =
1.00E-02
K5 =
7.41 E-07
11 K2 =
3.16E-02
K4 =
2.04E-03
«« =
4.27E-11
7.88E-04
2.76E-10
0.050
12
13 Species in charge balance:
14 [ H l =
3.62E-05
l[HAH =
15 [HeA z *] =
1.03E-13
[A'l =
16 [H 5 A*] =
2.84E-09
1^**] =
9.28E-10
[OK] =
[Na>
6.39E-05
[H4A] =
2.48E-06
17 [H : i A] =
2.99E-02
[HL] =
7.03E-07
6.84E-04
iH2n=
I
[L) =
18 [ H j A ' l =
3.85E-02
2.94E-13
19 Positive charge minus negative charge -9.77E-17
20 Formulas:
21 B10 = 10 A -B4 with analogous formulas for Kz - «e
pH =
4.441 <- initial value
is a guess
22 E7 = 10-E4 with analogous formulas for K^ and KL3
23
24
25
26
27
28
29
30
31
33
34
35
36
37
38
39
B14 = 10 A -H18
|H14 = H7/B14
IH15 - H3
Denomi = $B$14 A 6+$B$14 A 5*$B$10+$ B$14 A 4*$B$10*$B$11+$B$14 A 3*$B$1( ) * S B $ i r $ E $ 1 0
+$B$14 A 2*$B$10*$BSir$l =$10*$E$1- l+$B$14*$B$10*$B$1 r $ E $ 1 0 * $ E $ 1 r SHS10
+$B$ 10"$B$1 r $ E $ 10*$E$i r $ H $ 1 0 * $ H $ 1 1
|
|
j
Denom2 = $B$14 A 3+$B$14 A 2*$E$7+$B$14*$E$7*$ES8+SES7 è $E$ 8*$E$9
B15 = $BS14A6*$B$3/D(înorr 11
B16 = $B$Í4 A 5*$B$10*S; B $ 3 iDenoml
B17 = $B$14A3*$B$10*3>B$11*SES10*$ î$3/Denom1
B18 = $B$14 A 2*$B$10'3 ÏB$ir$E$10*$E :$11*$B$3/ DencXT11
E14 = $B$14*$B$10*SB$11*$E$10*$E$ i r $ H $ i o * î >B$3/Denom1
E15 = $B$10*$B$11*$E 510*$E$11*$HS 10*$H$ir$BS3/Denom1
H16 = $B$14A4*$B$10*S>B$11*$B$3/Deiiom1
E16 = SB$14 A 3*$ES3/Denom 2
E17 = $B$14 A 2*$E$7*$E$3/[ )enom2
E18 = SE$7 *SES8*$E$9*$ES 3/Denom2
H17 = $B$14*SES7*$E$8*$E $3/Denom2
E19 = B14+ 2*B15+B16+ 2*E1 6+E17+H15-B17-2*B18-3*E14-4*E15-E18-H14
12-11.
(a) Fe 3+ + SCN" ^
Fe(SCN)2+
[Fe(SCN)2+] = ß,'[Fe3+][SCN-]
Fe 3+ + 2SCN" ^
Fe(SCN)|
[Fe(SCN)5] = ß2[Fe3+][SCN"]2
Fe3+ + H 2 0 ^
FeOH2+ + H+
[FeOH2+] = A¡[Fe3+]/[H+]
H20 ^
H+ + OH-
[OH"] = A¿/[H+]
153
Advanced Topics in Equilibrium
ß
Yc-j+TcriJ= ß l vY
2+
Fe(SCN)
A,„
MV —
K V.
^Fc3+^SCN"
Y fav
.
ß
2
-
Ke(SCN)2
P, = I Q 3 «
,
* i -- * • Y
Y Fc 3 +
FeOH2+7HH
ß 2 = 1Q4.6
= 10-2.195
Ka
YH+YOH-
(b) Charge balance: [OH"] + [SCN~] + [N0 3 ] =
[H + ] + 3[Fe3+] + 2[Fe(SCN) 2+ ] + [Fe(SCN)J] + 2[FeOH 2 + ] + [Na + ]
(c) Mass balances:
Total iron = F F e = 5.0 mM = [Fe 3 + ] + [Fe(SCN) 2+ ] + [Fe{SCN)5] + [FcOH 2+ ]
Total thioeyanate = F SC N = 5.0 p.M - [FeiSCN) 2 *] + 2[Fe(SCN)J] + [SCN"]
[Na + ] = 5.0 uM
[ N 0 3 ] = 3(5.0 mM) + 15.0 mM = 30.0 mM
(d) [Fe(SCN)2+] + 2[Fe(SCN)$] + [SCNT] - F S C N
ßJ[Fe 3+ ][SCN-] + 2ß2[Fe 3+ ][SCN-] 2 + [SCN-] - F SC N
[Fe 3+ ](ßl[SCN-] + 2ß2[SCN-] 2 ) = F S C N - [ S C N - ]
F
.
[Fe3t]
SCN-[SCN-I
ßi[SCN _ ] + 2ß2[SCN-] 2
(e) [Fe 3+ ] + [Fe<SCN) 2+ ] + [Fc(SCN)$] + [FeOH 2+ ] = F F e
[Fe 3 + ] + ßi[Fe 3+ ][SCN-] + ß 2 [Fe 3 + ][SCN-] 2 + A;[Fe 3 + ]/[H + ] = F F c
[W]
K'[F¿+]
-
FFe - [Fe**] - ßi [Fe 3 + ][SCN]
K a [Fe 3 + ]
or[H+]
ß ^ F e 3 + ][SCN"] 2
— T — T T — T T : (which is easier to use)
FFe - [Fe 3 + ] - [Fe(SCN) 2+ ] - [Fe(SCN)$] v
(f), (g), (h), (i) The spreadsheet on the next page shows the following results:
a
8
[Fe *]
|[H1
[OH]
13 [SCN]
9.20E
14 2.03E-06 4.20E-03 1.58E-02
F
2
I
G
[FeSCN *] [Fe(SCN)2*] [FeOH *]
2.97E-06
1.06E-10
I
H
¡Net charge
8.02E-04I
-2.78E-17
Ionic strength = 0.043 4 M
pH-1.88
{[Fe^lT^mSCN-] = 293 (The ^
(j)
in the teXtb00k giVCS 270)
The next spreadsheet shows the results when the solution also contains 0.20
MKNO3:
|
154
Chapter 12
I
I
H
J-'*I
[Fe-]
[Hi
[FeSCN'*] [Fe(SCN>/] [FeOH^
j
Net charge
[OH]
13 [SON']
14 2.81E-06 4.45E-03 1.55E-02
1.18E-12" 2.19E-06' 6.82E-11
5.46E-04| O.OOE+QO
Ionic strength - 0.243 9 M
pH =1.94
[Fe(SCN)2+1
{[Fc3+] + rpeOi \21 j j [SCN"]
=
' 5 6 *Thc g r a p h
in t h e t e x t b o o k
8,ves • 5 0 )
Spreadsheet for 0 M KN0 3 :
A
B
C
D
1 Composition of Fe(lll)-thiocyanate solution
2
3
logß,=
3.03
1.07E+03
Pi =
4
logp2 =
4.6
lh =
3.98E+04
5
logK^
-2.195
Ka =
6.38E-03
6
logKw =
-14.00
Kw
=
E
F
1.75E+02
F* =
5.0E-03
IV =
1.94E+03
F sc .j =
5.0E-06
1.90E-03
[Na*] =
5. OE-06
1.83E-14
[N03>
2.3E-01
[Kl =
2.0E-01
K*'
=
7
6
9
Davles activity coefficients:
Fe 3 *
10 Fe(SCN)24
11 Fe(SCN),'
12
20
0.07
FeOH 2 t
0.30
OH"
0 74
0.30
0.74
SCN"
0.74
H*
0.74
IFel
13 [SCN]
14
15
16
17
18
19
H
pr =
K.' =
1.00E-14
G
2.81E-06
[Hi
4.45E-03
1.55E-02
Ionic strength =1
[OH]
[FeSCN2*]
1.18E-12
2
[FetSCNfel [FeOH *]
2.19E-06
6.82E-11
Net charge
5.46E-04
<-- Initial valu e i s O
0.24391
[FeSCN 2+ J/({[Fe 3 >[FeOH 2 1}[SCN ])=
New ionic strength =
pH=
1.94
156
[Total Fe = 5.OO0E-03 = [Fe3*] + [FeSCN2*] + [Fe(SCN)2+] + FeOH z 1
Total SCN = 5.00OE-06 = [SCN ] + [FeSCN2*] + 2[Fe(SCN) 2 1
21
22 Activity coefficients = 10*(-O.51*ch argeA2*(SQRT($C$16)/(1 *SQRT($C$1 6)>0.3*$C$1 6))
K,' = D5*B9/(E9*H10)
23 ß,' = D3*B9 *E10/B10
1
A
24 [V = D4*B9'E10 2/B11
1
K¿ = D6/(H9*H10)
25 F e n - (H4-A14V(F3*A14+2*F4*A14A2)
26 [H*] = F5*B 14/(H3-B14-E14-F14)
¿
27 |FeSCN '] :=F3*B14*A14
[OH] = F6/C14
[Fe(SCN) 2 1 = F4*B14*A14A2
28 [FeOHn=F5*B14/C14
pH=-LOG10(C14'H10)
29 Net charge = 3*B14+C14+ 2*E14+F14+2*G14+ H5-A14-D14-H6+H7
30 New ionic strength = 0.5'(H5+H6+A14+9*B14+C14+D14+4'E14+F14+4*G14+H7)
0.00E+00
155
Advanced Topics in Equilibrium
Spreadsheet f o r 0.20 M K N 0 3 :
log K, =
5
6
log Kx =
-2.195
«3 =
-14.00
=
K«
7
8 Davies activity coefficients:
3
0.07
9 Fe *
2
10 Fe{SCN) '
11 Fe(SCN)/
12
0.30
0.74
F
E
A
B
C
D
1 Composition of FeilllHhiocyanate solution
2
1.07E+03
3
3.03
logpi =
Pi =
3.98E+04
log | i 2 =
4.6
4
p2 =
6.38E-03
1.00E-14
H
G
Pi" =
1.75E+02
FFe =
5.0E-03
P2' =
Ka' =
1.94E+03
FSCN =
5.0E-06
1.90E-03
(Nal =
5.0E-06
1.83E-14
[N03] =
2.3E-01
[Kl =
2.0E-01
K*'
=
FeOH 2 *
0.30
OH"
0.74
SCN"
0,74
LH*
0.74
24
[FeSCN2*] [Fe(SCN)2*] [FeOH )
Net charge
[OH]
[Fei
[Hi
13 [SCNl
6.82E-11
5.46E-04
O.OOE+00
2.19E-06
1.18E-12
14 2.81 E-06 4.45E-03 1.55E-02
15
Ionic strength =|
0.2439 <-- Initial value is 0
16
[FeSCN2+J/( [Fe 3 >[FeOH 2 ']}[SCNl)=
0.2439
17 New ionic strength =
156
1.94
pH =
18
3
2
Total Fe = 5.000E-03 = [Fe *] + [FeSCN *] + [Fe(SCN)¡1 + [FeOH' ]
19
2
Total SCN = 5.000E-Û6 = [SCNl + [FeSCN *] + 2[Fe(SCN)i1
20
12-12.
(a) La 3 + + S0 2 4
La(S04)+
^
[La(S0 4 ) + ] = ßi'[La 3+ ][S0 2 4 -]
La 3 + + 2S0 2 4 - "^ La(S0 4 )2
[La(S0 4 )¿] = ß 2 [La 3 + ][S0 2 4 -] 2
La 3 + + H 2 0 ^
[LaOH 2 + ] = A-¡[La 3+ ]/[H + ]
H20 ^
LaOH 2 + + H+
7
ßi - ß.
L
K-a,
W —
[OH"] - A ^ [ H + ]
H+ + OH"
La3+YSQ24
Y
La(S04)+
A',H
* ! > * & *
Y
.
Y La 3 +
T
La(S04)¿
ßj = 10364
p 2 = 1Q5.3
LaOH2+V
K9 = 10-85
YH+YOH"
Charge balance:
[OH"] + 2[S0 2 4 -] + [ U ( S 0 4 ) 2 l = [H + ] + 3[La 3 + ] + [ U ( S 0 4 ) + ] + 2[LaOH 2+ ]
Mass balances:
Unthanum = F
u
= 2.0 mM = [La 3 + ] + [La(S0 4 ) + ] + [La(S0 4 )¿] + [LaOH 2+ ]
Sulfate = F S o 4 = 3.0 mM = [La(S0 4 ) + ] + 2[La(S0 4 )¿] + [S0 4 _ ]
Express [La 3 + ] in terms of [SO4]:
[La(S04) , ]
+
2[La(S04)2] + [S024-] = F S o 4
ß;[U 3 + ][S0 2 4 -] + 2ß 2 [La 3 + ][S0 2 4] 2 + [S024-] = F S o 4
156
Chapter 12
[La3+](ßi[S024"] + 2ß2'[S024]2) = Fso 4 -[S0 2 4 -]
[La3+] =
F
SQ4-[S024-]
ßi[S024] + 2ß^[S024]2
Express [H+] in terms of [La3+] and [SO 4"]:
[La3+] + [La(S04)+] + [La(S04)2] + [LaOH2+] = F La
[La3+] + ß;[U3+][S024] + ß2[La3+][S024-]2 + A;[La3+]/[H+] = F u
<[La 3+ ]
[H+] =
S
-¡
F U - [La3+] - ß,'[U3+][S024 ] - ß2[La3+][S024"]2
<[La3+]
or [H ] - (which is easier to use)
FLa - [La3+] - [La(S04)+] - [La(S04)2]
The spreadsheet is shown after (e).
(b) If La2(S04)3 were a strong electrolyte, p = \ {[La3+] • (+3)2 + [SO4] • (-2)2}
= 2 {(2.0 mM • 9) + (3.0 mM • 4)} = 15.0 mM. The actual ionic strength in
cellC17is6.3mM.
(c)[La3+]/FLa = 0.285
(d) We expected the solution to have a pH near neutral. pA'a for HSO4 is 1.99.
Therefore, wc did not expect very much HSO4 to be present. With a pH near
6, the fraction on sulfate that is protonated is -10 -4 .
(e) Evaluate the solubility product expression for La(OH)3:
[ U ^ H O H - f t r ^ Y ^ . = (5.69x 10-4)(1.04x 10-8)3(0.47)(0.92)3
= 2.3 x 10-28 < Asp for La(OH)3 = 2 x I0"21
La(OH)3(s) does not precipitate.
157
Advanced Topics in Equilibrium
A
B
C
D
1 Composition of La(lll)-sulfate solution
2
ßi =1 4.37E+03
3.64
3
iogßi =
1.59E+03
Fu =
2.0E-03
5.20E+04
Fsw =
3.0E-03
4
•09 32 =
5.3
82 =
2.00E+05
PV =
(y =
5
kKjK a =
-8.5
Ka =
3.16E-09
Ka' =
2.26E-09
1.00E-14
KJ -
1.18E-14
logK w =
-14.00
6
7
8 Davies activity coefficients:
9
La3*
K*
=
LaOH 2 *
0.47
2
SO., "
0.92
10 La(S0 4 )*
11 La(SO,)/
0.71
OH"
0.92
0.71
H+
0.92
0.92
12
13
14
15
16
H
G
F
E
[La;"*l
[so/-]
1.50E-03
[OH]
[H'J
5.69E-04
[La(S0 4 )1 [La(S0 4 )2l
1.04E-08
1.14E-06
1.36E-03
[LaOH21
6.69E-05
Net charge
3.12E-19
1.13E-06
Ionic strength =[_ 0.00629J<- initial value is u
17
New ionic strength =
0.O0629
18
pH =
5.98
Total La = 2.000E-03!
Total S O / ' = 3.000E-03
19
0.285
S0 4 2 1/F S 0 4 =
[La(S0 4 )1/F u =
0.681
0.501
[La(S0 4 ) 2 T/F La =
0.033
[La J ']/F La =
[LaOH 2 *]/F u =
5.6E-04
20
21
|
|
,
A
22 Activity coefficients = 'io*(-o.5i*charge 2*(SQR T($C$16V(1+SQRT($C$16))-0.3 $C$1 6 »
K^^DS'Bg/iEg'HIO)
23 ß,' = D3*B9*E10/B10
K*' = D6/(H9*H10)
24 py = D4*B9*E10*2/811
3
A
25 [La 1 = (H4-A14V(F3*A14+2*F4*A14 2)
26 [ H i = F5*B14/(H3-B14-E14-F14)
27 [La(S0 4 )1 = F3*B14*A14
[OH] = F6AÏ14
[La(S0 4 )z1 = F4"B14*A1* "2
2
pH = -LOG10(C14*H10)
28 ILaOH *] = F5*B14/C14
514-F14
;i4+E14+2*G14-2*A14-[
29 Net charge = 3*B14+C
30 New ionic strength = 0.5*(4*A14+9*B14+C14+D14+E14+F14+4*G14)
¡s
12-13.
CaS0 4 (s) ¿ P Ca 2+ + SO 4
ft ion pnîr
CaS0 4 (¿)
^
CaS04(a</)
Ca2+ + H20 £? CaOH+ + H+
A'hjiv
SO4" + H 2 0 ^
HSO4 + OH"
H 2 0 & H + + OH-
A sp = 2.4x10-5
fi.ion pair = 5.0
* 10"3
(A)
(B)
A a c i d = 2.0x l O ' 3
(C)
A W - 9.8 x lO" 3
(D)
A w = 1.0 x lO-"*
(E)
Charge balance: 2[Ca2+] + [CaOH+] + [H4] = 2[S024"] + [HS0 4 ] + [OH-]
(F)
Mass balance: [total calcium] = [total sulfate]
[Ca2+] + [ Ç a S e ^ ) ] + [CaOH+] = [S024"] + [HS04] + [ Ç a S O ^ ]
Write expressions for effective equilibrium constants K'.
(G)
158
Chapter 12
Ksp = [Ca 2+ ]y Ca 2 + [S0 2 4 ]Y S0 2- ^ KL
sp = "K.
sp
„
[CaOHMYCaOH+tH^YH^
„,
aC,d
2
_=>/ acid
"
[Ca + ]y Ca 2 +
"
2-,
7
2+
Ca Y S 0
2
= [Ca 2+ ][SOt]
(H)
4
?Ca2+
rCaOH+ÏÏH+1 _
4
= A:acid
YcaoH V"
[Ca 2 -]
®
_
[HSO¡JYHSO 4 -[OH-]YQHAbase ~
[S024]Yso2^•basc "~ Abase
YSO2-
[HSO;][OH]
YHSO4-YOH-
[so24-]
A„
Kw = [H+]yH+[OH-]yoH-
ftiu
'\v
— '
YH+YOH-
(j)
= [H+][OH-]
(K)
Substilute equilibrium expressions into mass balance (G):
[Ca2+] + [CaOH+] = [ S 0 4 ] + [HS0 4 ]
2+
] -_ [ S 0 2-]1 ++ ^ [ S p. jJ Q -_ [S0
r s o 2,f. + S ^
[Ca2 j + CidiCa
[H+]
4
[OH
4 ]^1 + [ 0 H . L
(L)
Substitute expression for [SO2"] from (H) into right side of (L):
!K
<cid[Ca 2+ ]
K's¡L
base
2+
[Ca ] +
+
2+ 1 +
[OH-];
[H ]
[Ca ]
Express [OH] in terms of [H + ]:
[Ca2t] +
Cid[Ca^
[H+]
K
- [Ca 2 + ]|/1 "+
[H + î|
A; J
¿ase
"basi
Multiply both sides by [Ca2+] and solve for [Ca 2+ ]:
(M)
In the following spreadsheet, we begin with a guess of pH = 7 in cell A14 and an
ionic strength of 0 in cell CI6. The spreadsheet computes the concentrations in
row 14 and the sum of charges in cell H14.
159
Advanced Topics in Equilibrium
C
A
B
1 Saturated CaSG,
2
|
|
2.4E-05
3
Kip =
|
2.40E-05
KiP =
5.00E-03Í
K.P-
5.0E-O3
5
Katja -
2.0E-13
K^'\
2.00E-13
6
—
9.8E-13
K,«,. =
9.80E-13
Kw=
1.00E-14
K* =
1.0E-14
7
8
9 Davies act vity coefficients:
24
1.00
10 Ca
+
11 CaOH
1.00
I
G
F
H
]
K„'=|
4
N)M(
E
D
so42
1.00
OH"
1.00
HS0 4
1.00
H*
1.00
12
13 pH
[Hi
[Ca2*]
[OH"]
[CaOHl
[S0 4 2 *]
[HS0 4 1
Net charge
3.82E-08
4.80E-08
4.90E-03
7.000Ó1 1.00E-07 1.00E-07
4.90E-03
9.80E-09
14
15
o.oooooi <- Initial value is 0
Ionic strength =
16
0.00000
17 New ionic strength =
18
A
19 Activity coefficients = 10^0.5rcharge 2*(SQRT($C$16y(1 *-SQRT($C$1 6))-0.3*$C$16))
K^' = B3/(B10*E10)
[ H i = 10 A -A14/H11
20
^ = 8 4
[OH] = D7/B14
21
I
22
K«« = B5*B10/(B11*H11)
23
KtM6 = B6*E10/(E11'H10)
24
K* ' = B7/(H11*H10)
[Ca 2 1 = SQFÎT(D3*(1+D6 *B14/D7)/{1+D5/B14))
[CaOH*]=D 5T314/B14
[S0 4 2 1 = D3/D14
[HS0 4 ]=D6*F14/C14
25
26 Net charge = 2*D14+E14+B14-2*F14-G14-C14
27 New ionic strength = 0.5*(B14+C14+4*D14+E14+4*F14+G14)
We then use Solver or Goal Seek to vary pH in cell A14 until the net charge in cell
H14 is close to 0. We set a limit of 1 E-18 for this calculation. The spreadsheet
computes a pH and new set of concentrations and an ionic strength in cell CI 7.
Wc transcribe the ionic strength from CI7 into cell C16 and repeat the cycle
several times until ionic strength no longer changes. Final results are displayed in
the following spreadsheet. The pH is 7.06 and the ionic strength is 0.041 M.
160
Chapter 12
A
B
1 Saturated CaS0 4
2
3
2.4E-05
Ksp =
C
D
K.P =
1.04E-04
4
Kp =
5.0E-O3
Kjp =
5.00E-03
5
"add
=
2.0E-13
K« w '=
1.39E-13
Ktjas*
=
9.8E-13
K*», =
6.80E-13
Kw =
1.0E-14
K=
1.44E-14
6
7
9 Davies activity coefficients:
10 Ca'*
0.48
S0 4 2
11 CaOH
HS0 4
0.83
E
F
H
G
0.48
0.83
OH"
0.83
H*
0.83
12
[Hl
13 PH
14
15
16
17
7.0648I
1.03E-07
1.39E-07
Ionic strength =|
New ionic strength =
12-14.
[Ca 2t ]
[OH1
[So42l
(CaOH*)
1.02E-02
1.37E-08
[HS0 4 ]
1.02E-02
Net charge
4.96E-08
8.20E-19
0.040711<- Initial value is 0
0.04071
AgCN(.v) ^
Ag+ + CN-
[Ag+] = AS^/[CN-]
HCN(í;í/)
CN" + H +
[HCN(ac?)] = [CN"][H + ]/AHCN
[AgOH] = AA'g[Ag+]/[H+]
^
Ag+ + H 2 0 ^
AgOH{aq) + H+
Ag+ + CN- + OH" ^
Ag(OH)(CN)-
[Ag(OH)(CN)-] = AA'g0HCN[Ag+][CN-][OH-] = AÁg0HCN<[Ag+][CN-}/[H+]
Ag+ + 2CN-
[Ag(CN)5] = f^[Ag+][CN"]2
Ag(CN)i
z
Ag+ + 3CN- F^ Ag(CN)v23-
H+ + OH"
H20 ^
K's p =
[Ag(CN)23-] = ß3[Ag+][CN-]3
Ksp_
YAg+YCN-
„<
^AgOHCN Y
[OH"]
KÛ
IICN
A,I I C N
K>
_
YCN-YH*
A.A2 -
Y
„
Ag + Y CN- 7 Oir
A A g OHCN Y
r
Ag(OH)(CN)-
Aw -
Ag +Y CN"
= A;/[H+]
KK!bsL
A An
v
KM
YH+YOII"
^Ag^CN"
ß2 = ß2
ß3 = ß3
Ag(CN)2
A-sp= 10-1566
AAgOHCN •' 1013-22
Y
Ag(CN)23-
A-HCN = 10-92»
ß 2 = 102<>-48
AAg=10-'20
ß 3 = 10217
Charge balance:
[OH"] + [CN"] + [Ag(OH)(CN)-] + [Ag(CN)¿] + 2[Ag(CN)23-]
- [H+] + [Ag+] + [K+] + [Na+]
(A)
Advanced Topics in Equilibrium
161
Mass balances:
[K+] = 0.10 M
{total silver }+ [K1] = {total cyanide}
[Ag+] + [AgOH] + [Ag(OH)(CN)-] + [Ag(CN)$] + [Ag(CN)23-] + [K+]
(B)
= [CN"] + [HCN] + [Ag(OH)(CN)-] + 2[Ag(CN)¿] + 3[Ag(CN)23]
which simplifies to
[Ag+] + [AgOH] - [Ag(CN)2] - 2[Ag(CN)23 ] + [K+] - [CN"] - [HCN] - 0 (C)
In the following spreadsheet, the initial ionic strength was guessed to be 0.10 M in
cell C23. [CN'] was then guessed in cell A17. [H+] and [OH"] in cells B17 and
B18 were computed from the known pH. [Ag+] in cell D17 was calculated from
[Ag+] = AYp/[CN-]. [AgOH], [Ag(OH)(CN)-], [Ag(CN)¿], and [Ag(CN)23-] were
computed from [CN - ], [H + ], [Ag+], and [OH-] with equilibrium expressions. The
mass balance Equation C is evaluated in cell H21. In the key step, the value in cell
H21 is set equal to 0 by using Solver to vary [CN"] in cell A17. When the mass
balance is satisfied, all concentrations must be correct. [Na+] is then computed in
cell C22 from the charge balance Equation A and the new ionic strength is found
in cell C24. The new ionic strength is then entered in cell C23 and the process is
repeated until the ionic strength no longer changes. The spreadsheet shows the
final concentrations when the mass balance is satisfied.
162
Chapter 12
A
B
C
1 Species in silver-cyanide solution
2
iogK„ p =
3
-15.66
K*
4
logKHa4 =
log KA, =
5
-9.21
KHCN
-12.0
6
I 0 9 KAt)OHCN =
13.22
7
log 82 =
20.48
13 Ag(CN) 2 "
0.77
2
14 Ag(CN), "
15
16
17
22
23
24
6.17E-10
F
"V-
G
3.65E-16
[Kl =
0.10
1.03E-09
PH =
12.00
1.00E-12;
KA[; =
1.00E-12
I.66E+I3I
Knew
KA^HCN»
9.95E+12
IV =
1.81E+20
ß3=|
5.01 E+21
Ps =
5.01 E+21
K*=|
1.00E-14
~
1.67E-14
=
H
=
3.02E+20
Ag(OH)(CN)
CN"
KH'
0.77
OH
0.77
0.77
H4
0.77
0.36
[CN"]
[Hi
1.513E-06 1.29E-12
[OH]
1.29E-02
18
19
20
21
2.19E-16
=
E
02 =
KACJOHCN
21.7
•08ßj =
logK^s
9
-14.00
10
11 Davies activity coefficients:
0.77
=
KA0 =
8
12 Ag*
D
[Agi
2.410E-10
[Ag(CN)2l
0.09999
[AgOH]
1.867E-10
[Ag(CN)/-]
4.187E-06
[Ag(OH)(CN)]
4.688E-05
[HCN]
1.901E-09
Mass balance: [ A g i + [AgOH] - [Ag(CN)2~] - 2[Ag(CN) 3 2 '] + [ K l - [CN") - [HCN] = -1.5E-17
0.01296 (from charge balance)
[Nal =
<- Initial value Is 0.1
Ionic strength = F
New ionic strength =
0.113 <- Substitute this value in lo C23 for next iteration
Total Ag + K* =
0.20004 check
Total CN =
0.20004 check
25
26
27
I
A
28 Activity coefficients = 10 ( •0.51*chargÉ»A2*(SQRT($C$23)/(H-SClRT($C$23))-0.3-$C$23)
IKHCN' = D4/ E13*H13)
29 I V = D3/(B12»E13)
^ • = 05*612^13
30 KäJOHCN' = D6»B12*E 13*H 12/E12
ß2' = D7'B12*E13 A 2/B13
|V = D8*R12"E13 A3/B14
31 Kw' = D9/(H12*H13)
32 [H + ]=10 A -H4/H13
33 [Ag*J= F3/A17
[OH] = F9/B17
I
34 [Ag(OH){CN)"] = F6*D17*A17*C17
2
[AgOH) = F5*D17/B17
A
35 [Ag{CN)3 "] = F8*D17*A17 3
[Ag(CN)2'] = F7*D17*A17 A 2
[HCN] = A17*B17/F4
36 Mass balance = D17+E17-D19-2*E19+H3-A17-F19
37 [Na*] = C17+A17+F17+D19+2*E19-B17-D17-H3
38 New ionic strength = 0.5*(A17+B17+C17+D17+F17+D19+4*E19 +H3+C22)
163
Advanced Topics in Equilibrium
12-15.
Fc 2 + + G" ^
FcG +
[FeG + ] = ßi[Fe 2 + ][G-]
Fe 2 + + 2G- === FcG2(aq)
[FeG 2 (^/)] = ß 2 [ F c 2 , ] [ G 1 2
Fe 2 * + 3G- ^
[FeG 3 ] = ß 3 [Fe 2 + ][G-] 3
FcG3"
Fc2+ + H 2 0 ^
HG ^
G" + H +
H2G+ ^
[HG] = [G-][H+]/A-2
[H 2 G + ] = [HG][H + ]/A¡ -
HG + H+
H 2 0 v* H + + OH"
YFe2+YG-
ßi-ßl T T
FcG +
Y 2
Fe +
- J^a »
ßl = 1 0 4 í l
A a = 10-9.4
[OH"] = A¿/[H + ]
Y Fe 2 + 7 2 G -
ß2 = ß 2 ^
r
k-i
[FeOH + ] - < [ F e 2 + ] / [ H + ]
FcOH+ + H+
r
FeG 2
jmG_
„,
„,
— Al
ß 2 = lO 7 - 65
Kx = j 0-2.350
7Fc2+Y3G-
ß3' = ß3T
r
A l
[G-][rl+]2/(K2K¡)
A l
-
A. i
FeG3
YH2G+
_ ^
A.,.,
ß3 = l O 8 8 7
jf2 = 10-9.778
Charge balance:
[OH"] + [G"] + [FeG3] + [CI"] =
[H + ] + 2[Fe 2 + ] + [FeOH + ] + [FeG + ] + [H 2 G + ]
(A)
Mass balances:
F F e - 0.050 M = [Fe2"1"] + [FeG + ] + [FeG2] + [FeG 3 ] + [FeOH + ]
(B)
+
F G = 0.100 M = [G'] + [HG] + [H 2 G+] + [FeG ] + 2[FeG 2 ] + 3[FeG 3 ]
(C)
2+
Substitute equilibrium expressions into the mass balance B to express [Fe ] in
terms of [G - ] and [H + ]:
F F e - [Fe 2 + ] + Pi[Fe 2 + ][G-] + ß 2 [Fe 2 + ][G-] 2 + ß 3 [Fe 2 + ][G"]3 + < [ F c 2 + ] / [ H + ]
FFe
rpe2+l =
—
(D)
2
+
1 + ß,'[G-] + ß 2 [G-] + ß 3 [G']3 + A-;/[H ]
In the spreadsheet on the next page, we guess a value for [G~] in cell A17 and
compute [H + ] = 10"PH/yH+ in cell B17. Equation D is used to find [Fe 2 + ] in cell
D17. Other concentrations in rows 17 and 19 are computed from [G~], [H + ], and
[Fe 2 + ]. Cell H24 checks that the sum of iron species equals F F e = 0.050 M. By
using the mass balance for iron to find Fe 2 + in cell D17, cell H24 must give F F e if
we have not made a mistake. We use the mass balance for glycine for the first
time in cell 1125, where we add all the glycine species. Then use Solver to vary
[G~] in cell A l 7 to satisfy the mass balance for glycine in cell H25. In Solver, Set
Target Cell H25 Equal To Value of 0J. By Changing Cells AJ7. [CI"] in cell C20
is then found from the charge balance equation A. The new ionic strength is
164
Chapter 12
computed in cell C22. Enter the value from cell C22 into cell C21 and repeat the
whole process several times until the ionic strength is constant.
A
B
C
D
2
1 Composition of Fe *-g ycine solution
2
3
logpi =
4.31
Pi = 2.04E+04
logß2 =
ß 2 = 4.47E-K37
4
7.65
togß3 =
8.87
5
03 = 7.41 E+08
kjgK.=
6
-9.4
Ka= 3.98E-10
7
8
log K, =
log K^ =
K,=
-2.350
-9.778
4.47E-03
K? = 1.67E-10
log K.,v =
9
-14.00
K=
10
11 Davies activity coefficients:
0.57
13 FeG*
0.87
14 FeG3
0.87
15
16
[Gl
[HI
17 1.10E-03| 3.64E-09
18 Guess
19
20
21
22
23
[Cl>
Ionic strength =
New ionic strength =
FcOH'
G"
H,G'
[OH1
[Fe' ]
3.64E-06
F
G
H
pH =
P i ' « 1.16E+04
P i " 1.91E+07
8.50
0.050
Fpe =
F6 =
ß 3 ' = 3.17E+08
K,= 2.60E-10
0.100
Ki' = 4.47E-03
K2' = 2.21 E-10
K,.,' = 1.33E-14
1.00E-14
12 Fe2*
E
1.33E-03
[HG]
1.82E-02
0.87
OH"
0.87
0.87
0.87
H*
0.87
[FeOH']
[FeG]
[FeGj]
[FeGal
9.50E-05
[H2G+]
1.48E-08
1.70E-02
3.10E-02
5.68E-04
0.0181 (from charge balance)]
0.0211 <- Initial value is 0.01 |
0.0211 <- Substitute this value into cell CîÏ1 for next ite ration
I
I
I
I
24
Mass balance for Fe: [Fe21 + [FeG*] + [FeG2] + [FeG3'] + [FeOH*] =
0.0500
25
Mass balance for G: [G ] + [FeG*] + 2[FeG2] + 3[FeG3"] + [H2G*] + [HG] =
0.1000
26
I
I
27 Activity coefficients = 10A(-0.51*chargeA2*(SC|RT($C$21J(/(1+SQRT($C$21))-0.3*Ï ÊC$21j)
28 pY = D3/(B12*E13) | fe' = D4/(E13*H13)
ß3' = D5*B' 2/H13
KW* = D9/(H12*H13)
29 K, = D6'B12-E13*H12/E12
KV = D7*B12*E13A2/B13
K2' = D8*B12*E13A3/B14
30 [H*] = 10MH3/H13
[OH'] = F9/B17
|[FeOHl = F6*D17/B17
7
A
A
31 [Fe *] = H4 r( 1+F3*A17+F4*A17 2+F5*A17 3+F67B17)
32 [FeG^] = F:}*D17*A17
[FeG2] = F4*D17*A17*2
FeG3"] = F5* 317*A17A3
33 [HG] = A17'B17/F8
[H2GT] = A17*B17A2/{F7*F8)
34 [Cf] = B17+2*D17+E17+F17+E19-A17-C17-H17
35 New ionic strength = 0.5*{A17+B17+C17+4*D17+E17+F17+H17+E19+C20)
165
Advanced Topics in Equilibrium
Fraction of Fe in each form:
[Fe 2+ ],2.7%; [FeG +], 34.0%; [FeG2], 62.0%; [FeG3], 1.1%; [FeOH+],0.2%
Fraction of glycine in each form:
[G"], 1.1%; [HG], 18.2%; [H 2 G + ], 0.000 015%; [FeG+], 17.0%; 2[FeG2],
62.0%; 3[FeG3], 1.7%
The chemistry: Wc dissolved FeG2 and found that the principal species arc FcG\
FeG2, and HG. The chemistry that requires HCl to be added to obtain pH 8.5 is
FcG2 T* FeG+ + G" followed by G" + H + ^
HG. The base G" is released
when FeG2 dissolves, so HCl is required to neutralize the base.
12-16.
(a) A range of initial values of pA'i and pA'2, such as 6 and 6 or 10 and 10,
converge to the correct solution. Even choosing a ridiculous value for pA¿„
such as 10, converges to the correct value after executing Solver more than
once.
(b) Fixing pA¿ at 13.797 and using Solver gives the optimized values of pK\ and
pA'2 - 2.312 and 9.630, which arc hardly different from the values obtained
when pA¿, is allowed to vary. However, inspection of the following curves
shows that nn(mcasurcd) deviates systematically from «H(theoretical) at the
end of the titration when /;n( measured) should approach 0.
Disagreement between
measured and theoretical
»H above pi I 11 when
pA\;= 13.797
5 0.8
measured
0.2
0.00
10.5
10.8
11.1
11.4
166
Chapter 12
•(a)
nH
moles of bound H+
-- total moles of weak acid
=
=
+ 2[H 2 A 2+ ] + [HA+]
[H 3 A ] + [H 2 A 2+ ] + [HA+] + [A]
3[H3A3+]
3+
or /J|IFH 3 A - 3[H3A3+] + 2[H 2 A 2+ ] + [HA+]
3+
2+
(A)
+
where FH 3 A = [H 3 A ] + [H 2 A ] + [HA ] + [A]
charge balance:
[H+] + [Na+] + 3[H 3 A^] + 2[H 2 A 2+ ] + [HA+] = [OH"] + [C1-]HCI + [CI-]H 3 A (B)
= "HFH 3 A from Eq. (A)
= 3FH 3 A
where [Cl"]Hci is from HCl and [CP]H 3 A is from H 3 A 3+ .
Each mol of H 3 A 3+ brings 3C1", so [C]-]u3A = 3 F H J A
We can rearrange Eq. (B) to solve for WR:
[H+] + [Na+] + WHFH3A = [OH'] + [C1-]HCI + 3FH 3 A
«HFH 3 A = 3FH 3 A + [OH"] + [CTJIICI
[QH-] + [Cl-]HCI-[H +]
"H = 3 +
1%A"
,
[H+] - [Na+]
[Na+]
(Ç)
Expression (C) is the same equation derived in the text, with n = 3.
The expression for OH (theoretical) is /^(theoretical) = 3 a H 3 A + 2 a H , A +
aHA(b) Optimized values arc pA¿ = 13.819, pAj = 8.33, pA2 = 9.48, and pA3 = 10.19
in cells B9.B12 in the following spreadsheet. These were obtained from initial
guesses of pA¿ - 13.797, pK] = 8, pA2 = 9, and pA3 • 10, after executing
Solver to minimize the sum of squared residuals in column K. The NI ST
database lists pA| = 7.85, pA'2 - 9.13, and pA3 = 10.03 at u • 0 and pA'i 8.42, pA2 = 9.43, and pA3 = 10.13 at p - 0.1 M. Our observed values at u =
0.1 M are in reasonable agreement with the N1ST values.
167
Advanced Topics in Equilibrium
K
G
H
I
J
A
I
B I
C
I
D
|
E
I F
Difference plot for tris(2-amirK>ethy1)amino
D17=10*-SBS9..'C17
jC17=10 A -B17/SB$8
2
|E17 = $B*7+($BS6-$B$3*A17-(C17-D17)'($B$4+A17)y$B$5
3 Titrant NaOH = 0.4905 C b (M)
denom = $C17A3+$C17A2*$E$10+$C17*$E$10*$E$11 +$E$10"$E$11*$E$12
40 V , (mL)
4 Initial volume =
1
5
6
7
S
9
HjAHCI added =
Number of H*=
Activity coeíf =
pK„' =
10 p K , 11 pK; =
0.139
0.115
3
0.78
L (mmol)
A (mmol)
n
YH
13819
8.334
9483
10.188
12 p K í 13 E(res¡of =
F17 = $C17' 3/denom
G17 = $C17 *2'$E$10/d<»nom)
H17 = $C17*$E$10*$E$ 11/denc m
117 = SES10"$EJ11 *$E$12/donom
J17 = 3*F17+2*G17+H17
K,=
Ka =
4.636E-09 = 10*-B10
K,«
6.485E-11 = 1 0 * 6 1 2
3.289E-10 = 10*-B11
0.0510 - sum of column K
14
15
v
PH
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
mLNaOH
000
2.709
IHT =
I0H1-
Measured
<IO*Vï* (lo^^yfH*]
9
2 51E-03
7JM
6.06E-12
R 'i'iF-l?
•
«fOA
«H2A
«HA
2
Theoretical (residuals) =
CIA
OW,-n»«,)
r»H
3.000
0011303
3000
0.008046
HH
3.106
3 090
1.000 0.000 0.000 0.000
1 000 0000 0 000 0000
•^^^^^-
8.158
8.283
8.91E-09
6.68E-09
1.70E-06
227E-06
2.628
2.558
0.649 0.338 0.012 0.000
0.579 0.401 0020 0.000
2.637
2.558
0.000077
0.000001
U.54
0.56
a.oHr
9.158
1 05E-09
891E-10
1 45E-05
1.70E-O5
1.926 0.145 0.641 0,201 0.012
1.856 0121 0.630 0.232 0.017
1655
0000002
0.78
0.80
9.864
9.926
1.75E-10
1.52E-10
8.66E-05
9.98E-05
1.10o| 0.010 0.277 0520 0 192
1.034 0 008 0.243 0.525 0.224
1.106
1.035
0.000031
0.000001
10 545
10.615
3.66E-11
3.11E-11
4.15E-04
4.88E-04
0.421 0 000 0.039 0.346 0.615
0.372 0.000 0.030 0.314 0.656
0.424
0.375
0.000011
0.000007
11.496
11.521
4.09E-12
3.66E-12
3.71E-03
3.93E-03
0.062 0.000I 0.001 0 059 0.940
0.057 DODO 0.001 0.056 0.943
0.061
0,058
0.000002
0.000000
0 34
0.36
:
:
•
1.00
1.02
:
1.38
1.40
1
^^^^^™^^^^^™
W,». •
3.0
•
t
n (obs)
^ 2.0
£
«
g 1.5
E
1.0
0.5
;
2.5
i • • 1l 1l l1 ••• •1 •
n (theory)
^^
7
PH
10
11
12
Chapter 12
(c) The fractions of each species arc computed in columns F, G, H, and I
beginning in row 17 in the spreadsheet. Results are shown following graph.
c
0.9
0.8
0.7
0.6
H,A
HjA 2*
HA
o
'S 0.5
I 0.4
0.3
0.2
0.1
0.0 5
•-,
\
y
6
7
8
9
10
11
•**
12
PH
12-18.
(a) From the equilibrium expressions, we write
[CuS04(aq)] = Ai'p[Cu2+][S024-]
[HSO4] = [SO 2 4 -][H + ]/A;
[CuOH+] = ßi[Cu2+][OH-]
[Cu(OH)2(flï)] - p2[Cu2+][OH-]2
[Cu(OH)3] - ß^[Cu2+][OH"]3
[Cu(OH)24-] = ß4[Cu2+][OH"]4
[Cu2(OH)3+] = ß,'2[Cu2+]2[OH-]
[Cu2(OH)22+] = ß2'2[Cu2+]2[OH-]2
[Cu3(OH)24+] = ß4'3[Cu2+p[OH-]4
Mass balances (F = 0.025 M):
Sulfate: F = [S024 ] + [HS0 4 ] + [CuS04(aq)]
Copper: F = [Cu2+] + [C\xS04(aq)] + [CuOH+] + [CU(OH) 2 (ü<?)] +
[Cu(OH)3] + [Cu(OH)24 ] + 2[Cu2(OH)3+] + 2[Cu2(OH)22+] + 3[Cu3(OH)24+]
Charge balance if the pH is not adjusted:
[OH"] + 2[S024 ] + [HS0 4 ] + [Cu(OH)3] + 2[Cu(OH)24 ] = [H+] + 2[Cu 2+ ]
+ [CuOH+] + 3[Cu2(OH)3+] + 2[Cu2(OH)22+] + 2[Cu3(OH)24h]
Substitute equilibrium expressions into the sulfate mass balance:
F = [ S 0 4 ] + [HS0 4 ] + [CuS04(a<7)]
...
Advanced Topics in Equilibrium
169
= [S024-] + [S0 2 4 ] [ H + ] / A ; + A ; P [ C U 2 + ] [ S 0 2 4 ]
2
F
or [S 4 ] =
° "
l+[H+]/AVA-i'p[Cu2+]
(A)
Therefore, we can find [S0 2 4 ] if we know [Cu2+] and [H + ]. We can also find
[HS0 4 ] from the equilibrium relationship [HSO4] = [S02¡][H+]/K'a. We find
the concentrations of copper species from their equilibrium relationships.
In the spreadsheet on the next page, pH is input in column A beginning at
cell A14. [H+] and [OH"] are computed from pH in columns B and C. The
initial value of [Cu2+] in each row of column D is a guess. We use Solver later
to find the correct value of [Cu 2+ ]. [S024 ] in column E is computed with
Equation A. The remaining concentrations in columns F through N are
computed from the equilibrium relationships. The sum of copper species is
tallied in column O. The key step is to use Solver to vary [Cu 2+ ] in column A,
so the total copper in column O equals 0.025 M. Solver must be applied
separately to each row of the spreadsheet.
(b) Column P gives the sum of all charges. This sum will be zero at the pH of
0.025 M Q1SO4, to which no acid or base has been added. Trial-and-error
variation of the pH shows that the charge is closest to zero at pH 4.61. Column
Q was not necessary for the problem, but it shows that the ionic strength of the
solution is near 0.075 M at most pH values, if no solids precipitate.
(c) To find out if the solubility of any salt has been exceeded, wc evaluate the
reaction quotient [Cu2+][OH~]2 for Cu(OH)2(» and CuO(.s) and the reaction
quotient [Cu 2+ ][OH-] 3/2 [S0 2 4 jW for Cu(OH)|.5(S04)o.250)- The solubility of
Cu(OH)i.5(S04)o.25(i) is exceeded above pH « 4.5. The solubility of CuO(s)
is exceeded above pH « 5. The solubility of CU(OH)2(.ï) is exceeded above pH
« 5.5. We predict that 0.025 M CUSO4 is not a stable solution. At the
calculated pH of 4.61, some Cu(OH)i,5(S04)o.25Í-s) will precipitate.
170
Chapter 12
A
B
C
1 Copper-sulfate-hydroxide system
2
F=
0.025 j M
log I V =
3
Kp' =
1.26
4
logK,' =
-1.54
Ka' =
5
•ogßi' =
6.1
ßi' =
log IV =
6
11.2
ß2' =
7
iogß» =
14.5
8
togß4' =
15.6
log ß12' =
8.2
Pl2*
=
10
log ßZ2' =
16.8
ß22'
=
11
log I V =
33.5
IV =
pH
[H*l
3
4
4.61
5
1.3E-03
1.3E-04
3.1E-05
1.3E-Ö5
1.3E-06
1.3E-08
1.3E-12
6
8
12
J
K
F
G
1.8E+01
log K*/ (OH-SO«) =
-16,68
2.9E-02
tog K*,' (OH )=
-18,7
to9^'(0)
=
-19.7
i V (OH-SO4) =
2.1E-17
K,::-(OH) =
2.E-19
K»'(0)«
log KJ =
2.E-20
•V« =
1.62E-14
3.E+33
[Cu2*]
[OH1
1.3E-11
1.3E-10
5.2E-10
1.3E-09
1.3E-08
1.3E-06
1.3E-02)
E
1.E+06
2.E+11
3.E+14
4.E+15
2.E+08
6.E+16
w
IV-
9
12
13
14
15
Ib
17
18
19
20
D
VH+
ISO/'"]
0.018824
0.018665
0.018602
0.018476
0.014136
9.8E-05
3.3E-11
1.8E-02
1.9E-02
1.9E-02
1.9E-02
2.0E-02
2.5E-02
2.5E-02
M
L
B
C
D
N
E
0.78
[CuOH*] [Cu(OH)2]
[HSO4I
[CuS0 4 ]
8.0E-04
6.2E-03
3.0E-07
4.8E-13
8.3E-05
6.3E-03
3.0E-06
4.7E-11
2.0E-05I 6.3E-03I 1.2E-05
7.8E-10
8.3E-06
6.3E-03
2.9E-05
4.7E-09
8.8E-07I 5.1E-03
2.3E-04
3.6E-07
1.1 E-08
4.4E-05 1.6E-04
2.5E-05
1.1E-12
1.5E-11
5.2E-07
8.3E-04
O
Total
13 [Cu(OH) 3 ] [Cu(OH) 4 ? ] [CujíOH) 3 *] [Cu 2 (OH)^] tCu.,(OH).t2']
14
1.2E-20
1.9E-30
7.1E-07
5.4E-16
3.6E-09
1.2E-17
1.9E-26
15
/.OE-06
3.5E-07
5.3E-12
16
8.1E-16
5.2E-24
2.8E-05
5.8E-06
1.4E-09
1.2E-14
1.9E-22
17
6.8E-05
3.4E-05
5.1 E-08
9.0E-12
1.4E-18
4.0E-04
18
2.0E-03
2.3E-04
19
6.3E-08
1.0E-12
1.9E-06
9.7E-04
7.6E-03
2,1 E-02
3.3E-03
2.1E-15
20
1.1 E-08
2.8E-06
A
I
-13.79
=
12
22 ¡H*]
H
copper
0.0250000
0.0250000
0.0250000
0.0250OOO
0.0250000
0.0250000
0.0250000
F
G
Q
Ionic
P
Charge
balance
2.1E-03
2.0E-04
-1.0E-07
-1.5E-04
-5.6E-03
-3.2E-02
-9.0E-02
strength
0.075
0.075
0.075
0.075
0.074
0.067
H
1
0.073
A
B14=10 -A14/$G$11
[OH]
C14 = $G$10/B14
23 [Cu *] Enter guess and find value with SOLVER to make total copper in column 0 = 0.025
24 [ S O / ]
E14 = $B$2/(1+B14/$D$4+$D$3*D14)
[HSO/]
F 1 4 « E14*B14/$D$4
25 [CuSO,,) G14 = $DS3*D14*E14
[CuOH*] H Í 4 - $D$5*D14»C14
26 [Cu(OH)2]
114 = $D$6'D14 # C14 A 2
27 [ C u í O H ^
28 [Cu^OH), 2 ']
K14 = $D$8*D14*C14*4
M14 = $D$10'D14 A 2-C14 A 2
[Cu(OHJj"]
3
[CUj(OH) *]
[CU3(0H)42+]
J14 = $D$7*D14*C14A3
L14 = $D$9*D14A2*C14
N14 = $D$11»D14A3'C14A4
29 Total copper
014 = D14+G14+H14+I14+J14+K14+2*L14+2*M14+3*N14
30 Charge balance P14 = B14-C14+2'D14-2*E14-F14+H14-J14-2*K14+3*L14+2*M14+2*N14
31 Ionic strength
Q14 = 0.5*(B14+C14+4*D14+4*E14+F14+H14+J14+4*K14+9*L14+4*M14+4*N14)
171
Advanced Topics in Equilibrium
Xu2*
<0
O-T* T..O.
9
©-
>*l
O
-2
. . x - .x-- x- x- x2
CuS04(ag)
-3
/
/
-x-.»..
Cuj(OH)4 *
'*-.
i
HS0 4
CUï(OH) *
f -5
O
S
f
A " /
/
\/Cu(OHfc(aQ)
/
¿•' /CuOH* /
.'
A
•
/
\
t
\
VCuiOH), 2 "
/
-a
i Cu2(OH)22* /
/
/
Cu(OH)3" , / \
V-
/ /
.7
—/—i
\
sV
*
J
\,
A
T
*
' 1
10
7
•
\cu2t
/
4
\
11
12
PH
The following formulas were used when writing this problem to compute
equilibrium constants K' for p. = 0.1 M from equilibrium constants K for p = 0.
If A' was reported for another ionic strength, such as 1 M, it was first converted
to K for p = 0 and then converted from K to K' for p = 0.1 M. Davies activity
coefficients were used.
Y
^ip
=
Cu 2 + Y S0 2 ;
^ip y
CuS0 4
y
ß2 = ß2
Y
Ka - Ka Y
7
Cu2+iÔW
Y
Cu(OH)2
PÍ = ß3
so 2 ; 7 ii +
T
Cu(OH)3
V,.;OII
T
ß ; = ß4
Cu 2 + T OH'
Cu 2+Y OH"
Y
Cu(OH)24"
Y¿ 2+Y óV
á W
Cu 2 (OH) 3+
ßi - ß i
Cu 2 + Y OH"
Y 2+
PÍ2 - ßl2 T
Y
HSO;
ß 2 2 = ß22
y
Cu2{OH&
ß 4 3 = ß43 Y
Cu3(OH)24+
172
Chapter 12
A sp (UII-SU4) -
T
12-19.
/: sp (QH-S0 4 )
3/2„,|/4
vv3/2 ¥ l / 4 ,
'CV+'OH^SO2;
vy
Cu2+yOH-
W^oír
(a) [Na + ] + [NaT"] + [NaHT] = F N a = F H 2 T
Substitute [NaT"] = À-NaT-[Na+][T2-] and [NaHT] - A: NaHT [Na + ][HT-]:
[Na+] + A- N ar[Na + ][T 2 -] + K NaHT [Na + ][HT-] = F „ 2 T
[Na+]{1 + A-NaT-[T2-] + A-NaHT[HT-]} - FH 2 T
FH2T
]
^
- 1 + * NlT -[T2-] ^NaHTtHT-]
<A>
(b) [H2T] + [Hr] + [T2-] + [NaT] + [NaHT] = FH 2 T
Make the following substitutions to find expressions in terms of [T2"], [Na+],
and [H+]:
P™
=
A ^ n*T ¡
[HT] = ^
[T 2 -];
[NaT] = K NaT -[Na + ][T 2 -]
[NaHT] = A NaHT [Na + ][HT-] = ^NaHîtNa+f^ 1 [T2"]
Then put these expressions into the mass balance:
K% lj2'l
+
^
ÍT2"1
+
P*l + ^NaT-[Na+][T2-] + /ï N a H T [Na + ] I H ^ 1 [T 2 '] = F H 2 T
and solve for [T2"]:
[T
]
" ÍH+12
[H + ] "
/vve + V +
] f
~\W]
(B)
%aT-[Na + ]+^ NaHT [Na + ] L ^ i
(c) To find [HT-], make the following substitutions in the mass balance for H2T:
[H+l
•>
K2
[H2T] = ^ [ H T - ] ;
IT2"] = ¡ ¡ p j [ H r ] ; [NaHT] = K NaHT [Na + ][HT-]
[NaT"] = K NaT -[Na + ][T 2 ] = A N a T -[Na + ]¡^¡ [HT']
Then put these expressions into the mass balance:
^ M [Hr ] + [HT'] + ^
[HT] + K Nar [Na+]j^j [Hr] + KNaHT[Na+][HT-] = FH2T
and solve for [Hr]:
173
Advanced Topics in Equilibrium
tHT"J
=
ríFi
Y,
"2T
K2
'
(C)
K\ + l + [H+Ï + ^NaT-[Na+]7J^ +^NaHT[Na+]
To find [H2T], make the following substitutions in the mass balance for F\21:
K\
,
K\K2
2
[Hr] = ^
[H2T]; [T -] = p^2[H2T]
^]/C 2
[Nar] = A-Nar[Na+][T2] = K Na rl>la + jT^H2T]
[NaHT] =KNaHT[Na+][Hr] = *^ T [Na + ]jjp¡ [H2T]
Then put these expressions into the mass balance:
K\K2
K\K2
K\
K\
+
+
[H2T] + ¡ ^ [H2T] + ï^ïjflH 2 T] + ANaT-tNa ]i¡ITJ2[H2T] + K N a H T [ N a ] ^ [H2T]
= F
H2T
and solve for [H2T]:
F
[
R2T
1
=
H2T
—KX
KXK2
ZW2
~TT
+
1 + ÑP¡ + ¡J[+-J2 ^NaT-tNa+lyjT^ +KNaHT[Na+]7j^
<D>
(d) Insert equations A, B, C, and D into the following spreadsheet to compute
[Na+], [H2T], [HT-], and [T2_] in cells B12, H10, El 1, and E12. Excel
indicates a circular reference problem with these new formulas. In Excel 2007,
click the Microsoft Office button at the upper left of the spreadsheet. Click on
Excel Options at the bottom of the window. Select Formulas. In Calculation
options, check Enable iterative calculation and set Maximum Change to le-16.
Click OK. (In earlier versions of Excel, go to the Tools menu and choose
Options. Select Calculation and choose Iteration. Set the maximum change to
le-16 and click OK.) Guess a pH (such as 6) in cell H13. Select Solver and
Set Target Cell FA5 Equal To Value of 0 By Changing Cells HO. Click Solve
and your spreadsheet should find the concentrations in the spreadsheet on the
next page.
174
Chapter 12
A
B
C
D
E
F
G
1 Mixture of 0.020 M Na*HT, 0,015 M PyH'CI, and 0.010 M KOH
2 With some Na* ion pairs
3 FtCT0.020
[K>
0,015
Fp** =
4 pK,=
3.036
PK,=
5.20
Kw =
5 pK2 =
4,366
K9 =
6.31 E-06
KNST. a
6
K,=
9.20E-O4
7
K2 =
4.31 E-05
KNüHT
I
0.010
1.00E-14
8
1.6
=
I
8
9 Species In charge balance:
10 [ H i 5.45E-05
[OH] =
1.84E-10
11 [PyH*]=
12 [Na*] =
13 [K1 =
[HT]=
H*] =
[crj=
1.O0E-02
7.92E-03
0.0150
1.34E-02
0.0185
0,0100
H
14
Other concentrations:
IHjT] =
5.93E-04
[Py] =
[NaHT] =
pH =
1.56E-03
2.97E-04
4.264 <-- initial value
[NaT] =
1.17E-03
is a guess
15 Positive charge minus negative charge = | -3.69E-16|
16
E15 = B10+B11+B12+B13-E10-E11-2*E12- E13-E14
17 Check: [PIHi + [P] =
0.01500
18 Check: [H 2 r]+[HT>rn+[NaT +[NaHT] =
0.02000
19 Check: [Na *]+[NaT"]+[NaHT] =
0.02000
20 Formulas:
21 B6 = 10A-B4
B 7 = 1 DA-B5
E5 = 10A-E4
E10 = H4/B10
A
22 B10=10 -H13
B13 = H3
E13 = E3
23 E11 = B3/(B10/B6 + 1 + B7/B10 + H5*B12*I37/B10 + HI3-B12)
24 E12 = B3/((B10A2/(B6*EI7))+(B10/B7)+1+H£ >*B12+H6'EH2*B1<)/B7)
2b H10 = B3/(1 +B6/B10 + B6*B7/B10A2 + H5'B12*B6*B7/B10 A2 + H6*B12*B6/B10)
26 B12 = B3/( I + H5*E12 + H6'E11)
B11 = B10*E3/(B10+E5)
27 E14 = H5*EH2*E12
H11 =E5*E3/(B10+E5)
28 H12 = H6* J12*E11
29
30
31 Na distribuí ion (%)
32 [Na'] =
92.G
33 [NaT] =
34 [NaHT] =
35
36
5.9
1.5
H2T distribution (%)
[H2T] =
3.0
[HT1 =
P*] =
50.1
39.6
[NaT] =
5.9
INaHT1=
1.5
CHAPTER 13
FUNDAMENTALS OF ELECTROCHEMISTRY
13-1.
Electric charge (coulombs) refers to the quantity of positive or negative particles.
Current (amperes) is the quantity of charge moving past a point in a circuit each
second. Electric potential (volts) measures the work that can be done by (or must
be done to) each coulomb of charge as it moves from one point to another.
13-2.
(a)
1/1.602 176 53 x 10"19 C/electron - 6.241 50948 * 1018 electrons/C
(b)
F = 96485.338 3 C/mol
(a)
/ = coulombs/s. Every mol of02 accepts 4 mol of e". 16mol02/day
13-3.
= 64 mol e7day - 7.41 x \Cy4 mo\ e"/s = 71.5 C/s = 71.j A
(b)
/ = Power/£ = 500 W/l 15 V = 4.35 A, The resting human uses 16 times
as much current as the refrigerator.
U-4.
(c)
Power = E-1 = (1.1 V) (71. 5 A) = 79 W
(»
I = o2.0
f f x: °1(PW
? J w = 3 0 0 mA = 3.00 x 10-3 c / s
f
3.00* 10-3 C/s
j (6.022 x 10 23 e7mole) = 1.87 x 10 16 e7s
4
i9.649* 10 C/mol
(b)
P = E-I = (6.00 V)(3.00 x 10-3 A ) = 1.80 x 10' 2 W
1.87 x 10 l 6 e7s
13-5.
(c)
(1.87 x io 1 6 c7s)(l 800 s) - 3.37 x ]0 1 9 electrons = 5.60 x Ws mol
(d)
P = EI = E{E/R) = E2/R =>£ = y[PR = V ( 1 0 0 W)(2.00 « 103 W) = 447 V
(a)
I2 + 2e" ?* 21"
(b)
2S2O3" ^
(c)
1.00 g S2O37 (112.13 g/mol) = 8.92 mmol S2O2" = 8.92 mmol e"
(I2 is the oxidant)
S4O6" + 2e~
(S2O3" is the reductant)
(8.92 x 10-3 m o i ) (9.649 x io* C/mol) = 861 C
(d)
Current (A) = coulombs/s = 861 C/60s = 14.3 A
175
176
13-6.
Chapter 13
(a)
Oxidation numbers of reactants: N ( i n N H 4 ) CI (in CIO¡ ) Al
-3
+7
0
Oxidation numbers of products:
N(inN2)
0
CI (in HCl)
-1
Al (in AI2O3)
+3
NH4 and Al arc reducing agents and CIO4 is the oxidizing agent.
(b)
Formula mass of reactants = 6(FM NH4CIO4) + 10(FM Al) - 974.75
Heal released per gram = 9 334 kJ/974.75 g = 9.576 kJ/g
13-7.
In a galvanic cell, two half-reactions are physically separated from each other. At
the anode, oxidation generates electrons that can flow through the electric circuit
to reach the cathode, where a reduction occurs. The favorable free energy change
for the net reaction provides the driving force for electrons to flow through the
circuit. There must be a connector (such as a salt bridge) between the two halfcells to allow ions to flow to maintain electroncutrality.
13-8.
(a)
Fe(.y) | FeO(s) | KOH(aq) \ Ag 2 0(s) | Ag(¿)
FcO(s) + H 2 0 + 2e" ^
Ag 2 0 + H 2 0 + 2e"
(b)
Fc(s) + 20H"
^ 2Ag{s) + 20H"
Pb(j) I PbS04(.v) I K2S04(aq) \ \ H2SÖ4(aq) | PbS04(j) | Pb02(¿) | Pb(s)
PbS0 4 (i) + 2e" F* Pb(s) + SO2/
Pb0 2 + 4H + + SO4" + 2c" ^
13-9.
Fe 3 ' + c- ^
Fe 2+
Cr 2 0 2 " + 14H+ + 6e' ^
2Cr3+ + 7H 2 0
<oy
.
Cr2á¡
Fe3<
Fe2»
PbS0 4 (s) + 2H 2 0
•
Cr3*
HA
177
Fundamentals of Electrochemistry
13-10.
(a)
Zn 2+ + 2e" ^
Zn(s)
ET = -0.762 V
C12(0 + 2e" TA 2C1"
F «1.4V
The Appendix lists standard reduction potentials for C\2(g) and Cl2(fl<?), but
not for Cb(/). Both listed potentials are close to 1.4 V, so the potential for
C\2(l) is probably also close to 1.4 V. Electrons flow from the more negative
electrode (Zn) through the circuit to the more positive electrode (C).
(b)
One mol of CI2 requires 2 mol of e".
Moles ofCl2 consumed in 1.00 hr =
2
(mol of eThr) =
Í5Í1.OO x 103 jV(9.64 x 104 c / m o i ) (3 600 s/hr) = 18.7 mol of Cl 2 = 1.32 kg.
13-11.
(a)
anode: C6Li ^ C 6 + Li+ + e"
cathode: C0O2 + Li* + e* r* LiCo0 2
(b)
1 m A = l x lO" 3 - 1 h = 3 600 s
1 mA-h = ( l x l 0* j ) ( 3 600 s) = 3.6 C
(c)
s j g f a t o o i = 1.02i7 x IO2 mol LÍC0O2 which holds 1.02,7 * 10"2 mol Li+
and 1.02n * IO"2 mole\ (1.02,7 * IO"2 mol c)(9.649 * 1 0 5 ¿ ^ J = 985. 8 C
(
C
Y l mAh^i ^_„
mAh
273i!
Charge capacity = ^985.8 g LÍC0O2A 3.6 C J "
g LÍC0O2
(d)
„
140 mA-n/g
Fraction of Li available = 273 8 mAh/g
(e)
Energy stored per unit mass • work that can be done / mass = Eq I mass
=
Ä
„
= <3-7 v { 1 4 0 gTÎcoO-J = i 3 - 7 v >(°- ]40 ilMö-J^^il^fe
13-12.
CI2 is strongest because it has the most positive reduction potential.
13-13.
(a)
Since it becomes harder to reduce Fc(III) to Fe(II) in the presence of CN",
Fe(Ill) is stabilized more than Fc(II).
(b)
Since it becomes easier to reduce Fc(IH) to Fe(II) in the presence of
phcnanthroline, Fc(II) is stabilized more than Fe(lII).
178
Chapter 13
13-14.
E* applies when activities of reactants and products arc unity. E applies to
whatever activities exist. At equilibrium, E goes to zero. E° is constant.
13-15.
(a)
Zn(.s) | Zn2+(0.1 M) 11 Cu2+(0.1 M) | C\x{s)
right half-cell: Cu 2+ + 2e" ^ Cu(s)
E+ = 0.339 V
Zn 2 ' + 2c" ^ Zn(.v)
El = -0.762 V
left half-cell:
F / n r î û 0 0 5 9 1 6,
1 \
/
0.059 16,
1 \
lo
]o
E= | 0 . 3 3 9 j
ßÖTTi - j - 0 - 7 6 2 ¿
&0~ï ) =
L m
V
Since the voltage is positive, electrons are transferred from Zn to Cu. The
net reaction is Cu 2+ + Zn(s) ^ C\x(s) + Zn 2+ .
(b)
I M * (a)
(b)
Since Cu 2+ ions are consumed in the right half-cell, Zn 2+ ions must migrate
from the left half-cell into the salt bridge to help balance charge. I hope you
like Zn 2+ , because that is what your body will take up.
e=
_o.23S_aoploggÄ
«-««-Mp^uag--«™
Pu» I Br 2 (0 | HBr(aq, 0.10 M) || Al(N03)3Î<rç, 0.010 M) | Al(.v)
(b)
right half-cell:
Al 3+ + 3e" ^ AI(j)
E+ =—\.677 V
left half-cell:
Br2(/) + 2e" ^ 2Br" EL = 1.078 V
• u. i if n n
f . ^
0 0 5 9 1 6.
1
}
lo
right half-cell: £ + = | - 1 . 6 7 7 3
ë [ 0 . 0 1 0 ] / = -l-716 4 V
-~/
f o7'î
left half-cell: £_ = {1.078-
00S 916
2
log [0.10]2 } = 1.1372 V
E - E+ -TL = -1.7164 - 1.1372 = -2.854 V. The right electrode is more
f
'.(#77
negative, so electrons flow from Al to Pt. Reduction occurs at the left-hand
electrode. The spontaneous reaction is
2*1*5
f Br2(/) + Al(<?) == 3Br' + Al3+
(c)
14.3 mL of Br2 = 44.6 g = 0.279 mol of Brç. 12.0 g of Al = 0.445 mol of
Al. The reaction requires 3/2 mol of Br2 for every mol of Al. The Br2 will
be used up first.
(d)
0.231 mL of Br2 = 0.721 g of Br2 = 4.51 x 10-3 m o ] Br2 =
9.02 x IO-3 mole" »
870C
Work
= E.q
= (].50)(870) =1.31 kJ.
179
Fundamentals of Electrochemistry
/ =y[P/R = V(1.00* 10-4)/(1.20x IO3) - 2.89 x W4 A
(e)
- 2.99 x 10-9 mol e7s = 9.97 x IO' 10 mol Al/s = 2.69 x IO"8 g/s
13-18.
13-19.
The activities of the solid reagents do not change until they are used up. The only
aqueous species, OH-, is created at the cathode and consumed in equal amounts at
the anode, so its concentration remains constant in the cell. Therefore, none of the
activities change during the life cycle of the cell until something is used up.
0.059 16
(k) right half-cell: £ + = - f o . 2 2 2 - - ^
log [CI"]2} =0.281 2 V
left half-cell: £_ = {-0.350 E =
(b)
005
2
916
log [ F ] 2 } = -0.290 8 V
£ + - £ _ = 0.2812-(-0.290s) = 0.572 V
Electrons flow from the left half-cell (£ = -0.290g V) to the right half-cell
(£ = 0.2812 V).
[Pb2+] = K sp (for PbF2) / [F - ] 2 = (3.6 x IO 8 ) / (0.10)2 - 3.6 x 10-6 M
(c)
[Ag+] = Ksp (for AgCl) / [CF] = (1.8 x 10>°)/(0.10) = 1.8 x 10-9M
right half-cell: E+ = { 0.799 - ^ 2y ^ log y ^+ p } " 0 - 2 8 1 2 v
[Ag ]<
1
0.059 16
287 0 V
-0.126left half-cell: E.
log"
2
'"S [Pb2+j
}=-«.
-{
E =E+-E- = 0.2812 -(-0.287 0 ) = 0.568 V
The agreement between the two calculations is reasonable.
13-20.
A hydrogen pressure of 727.2 Torr corresponds to (727.2 Torr)/(760 Torr/atm)
0.956 8 atm.
(0.956 8 atmX 1.013 25 bar/atm) = 0.969 5 bar.
[0.010001(0.914)
0.7983= £- A g+|Ag-0.05916log ( 0 9 6 9 5) i/2 [0.01000](0.898)
=> £*Ag+|Ag • 0.7992 V
13-21.
Balanced reaction: HOBr + 2e~ + H + ^
HOBr — Í B r 2
2Br2
HOBr
E.
Br"
Br"
_ -1^.584)-1^.098) _ ,
Br" + H 2 0
AG¡ =-lM( 1.584)
AC?2= -1F(I.098)
AG3 = AC, + AG2 = -2FE3
34,
v
180
Chapter 13
13-22.
2X+ + 2c" ^
*J0_+
2e" ^
2X(.v)
EX = E2
X+
EL = E\
3X+ == X3+ + 2X(s)
E 3 = E 2 - E,
If E2 > E\, then £3 ¡> 0 and disproportionation is spontaneous.
13-23.
right half-cell: Cu 2+ + 2e" ^ Cu{s)
left half-cell:
E+ = 0.339 V
Ni 2+ + 2e" ^ Ni(j)
EL = -0.236 V
The ionic strength of the right half-cell is 0.0090 M, and the ionic strength of the
left half-cell is 0.008 0 M. At p - 0.009 0 M, yCu2+ = 0.690.
At p = 0.0080 M , YNj2+ = 0.705.
_.
„. 0.05916,
1
E+=E+
2
^ [ C u ^ y ^
0.05916
l
= 0 . 3 3 9 - — 3 — l o g ( 0 0 0 3 0 ) ( 0 6 9 0 ) = 0.2596 V
„
0.05916,
1
E =E
2
- -~
2
^ P
^
0.059 16.
1
n „ £
lo
= -0.236j
S (0.002 0X0.705)
E = E+-E- = 0.580 V
=
-°- 3 2 0 3 V
Electrons flow from Ni (E = -0.320 3 V) to Cu (E = 0.2596 V).
13-24
13 24.
(a) F = =*£- =
{a) L
nF
(b)
5.
13-25.
(a)
( + 257*lQ3j/mol)
( 2 ) ( 9 6 4 8 5 x]()4 c / m o | )
- 1.33 V
K = 10"£V0.059I6 = ] x 1045
4[Co3+ + e" ^
C¿"]
E'+ - 1.92 V
~2[50 2 + 2H + + 2c ^ H 2 0 ]
4Co:l+ + 2H 2 0
^ 4Co 2+ + O2 + 4Hf
AG' = -AFE° - -2.7 x 105 J
(b)
EL = 1.229 V
Ag(S203)32 + e"
- Fc(CN)^ + e
ET •- 0.691 V
K = 104r/0.059 16 = 1Q47
^ Ag(s) + 2S2023
Fe(CN)t
EL = 0.356 V
Ag(S203)32 + Fe(CN)t 5* Ag(s) + 2S2023- + Fe(CN)36_
AG* - -\FE* = 32.7 kj
El = : 0.017 V
ET
-0.339 V
K = 10^^05916 = 1.9 x 10-6
181
Fundamentals of Electrochemistry
13-26.
5Ce 4+ + 5e' ^
(a)
5Ce3+
EX = 1.70 V
' MnO;+ 8H+ + 5c" ^
Mn 2+ + 4H 2 Q
EL = 1.507 V
5Ce 4+ + Mn 2 + + 4H 2 0 ^
5Ce3+ + Mn0 4 + 8H+
£* = O.I93V
(b)
AG* = -SEE* = -93.1 U
(
0.05916 .
K = lO*^'0.059 16 =
[03+15 ï
f, „ _
2
x 10»^
[Mn2+1
0.059 16,
= 1.5223-1.5425 - -0.02 0 V
(d)
AG = -5FE = +10 kJ
(e)
0.05916
[Cc3+]5[Mn0 4 ][in 8
At equilibrium, £ = 0 => £* = " ~J~ log
rçc 4+ ] 5 [Mn 2 ' ]
=> [H+] = 0.62 => pH = 0.21
Sn 4+ + 2e" T* Sn 2+
13-27. right half-cell:
- 2 V 0 2 + + 4H + + 2e" ^
Sn 4+ + 2V3+ + 2H 2 0 ^
left half-cell:
net reaction:
2V 3+ + 2H 2 0
Sn 2+ + 2V0 2 + + 4H+
_
0.05916,
rV02+12[H+14[Sn2+1
E - E- log
rv3+]2[Sn4+]
2
_
nion
(0.116)2(1.57)4(0.0318)
0.05916,
-0.289 = F - " ^ — l o g
(o.hViO.O^lS)
=>
E
=
.,.,..
~°266
V
=> A: = io 2 ^/0.059 16 = 1.0 x 10-9
13-28.
Pd(OH)2(s) + 2e" ^
" Pd 2f + 2e' ^
Pd(s) + 20H"
PdQy)
Pd 2+ + 20H"
0.05916,
„
Butj^sp = 3 x IO"28 => £* =
2^
log/: s p
Pd(OH)2 í
EX
!•: - •-
0.915 V
E "= £1-0.915
0.814
-0.814 = E\ - 0.915 => £[ = 0.101 V
13-29.
Br2(/) + 2e" ^
2Br"
~Br 2 (íií) + 2e' T* 2Br"
O
/A
^
U
/
Ï
Br2(/) f* Br 2 (a?)
£+ = 1.078 V
EL = 1.098 V
£* = "O"020
V
0-05916,l o [Br2(«y)J
rt™
At equilibrium, £ = 0. Therefore, 0 = -0.020 2
ê [Br2(/)1
^
182
Chapter 13
[Bt2(aq)]
That is, the solubility of Br2 in water is 0.211 M = 34 g/L.
13-30.
FeY2"
FeY" + e" ^
EX
FeY" + c" T± Fe 2+ + Y4"
Fe 2+
4
EL = -0.730 V
2
+ y - =* FeY "
£* = £+ + 0.730
But £* = 0.059 16 lograr (for FeY2")] = 0.846 V=> EX = £* - EL = 0.116
V.
13-31.
£*(7) = E° +
^AT
£°(50* C) = -1.677 V + (0.533 x lf>3 V /K) (25 K) - -1.664 V
13-32.
1. 2CU(A) + 21" ?¿ 2CuI(í) + 2c'
AG] = +2£(-0.185) = -35.7 0 kJ
2. 2Cu 2+ + 4e" ^ 2CU(J)
AG2 = -4£(0.339) - -130. 8 3kJ
3. hydro ^ quinone + 2H+ + 2c"
AG3 =» +2f(0.700) = 135.oskJ
4. ( 1 )+(2)+(3): 2Cu2+ + 2T+ hydro =*
2Cul(i) + quinone + 2H
AG4 = AG] + AG2 + AC3
+
= - 3 1 4 kJ
-AG4
.
Since 2c arc transferred in the net reaction, £ 4 = 2 JT " +O.I63 V
K = 102<0.I63V0.059I6 = 3. 2 x
13-33.
(a)
10 5,
^-(right)
RT
¿(right) = f r i g h t ) - 2 £ m p l S ( r i g h t )
RT
J^-ileft)
Net reaction: reverse left half-reaction and add it to right half-reaction:
MgF20?) + A1 2 0 3 (A) + Vi 0 2 (g) + 2e- ^ MgAl 2 0 4 (s) + 2F"
MgO(s) + 2F- ^
nct reaction:
AI2O3CÏ) + MgO(í) ^
MgF2(5) + Vi 02(g) + 2eMgAl204(ó)
Nernst equation for net reaction:
RT
£(r,ght)-£(left) = f r i g h t ) -£-(left) - ^
^lF2-(right)
^ 7 ^ +
RT
j2L2(left)
§ l n ^ ^
183
Fundamentals of Electrochemistry
The activities of F" are the same on both sides and the activities of O2 arc
also the same on both sides, so the In terms cancel, leaving £(cell) = £°(right)
-F(left) = £*(cell).
(b)
AG' = -nFET = -(2)(9.648 5 x IO4 C/mol)(0.152 9 J/C) = -29.51 kJ/mol,
where we made use of the fact that a volt is equivalent to one joule/coulomb.
(c)
AG° = AH" -TAS'
-nFET = A/f -FAS* = -«£(0.122 3 + 3.06 x IO"5 7)
- -«£(0.122 3 V) - «£(3.06 x IO"5 T)
= -«£(0.122 3 V) - T {«£(3.06 x 10"5 V/K)}
AIT
AS*
A/T = -n£(0.122 3 V) = -(2)(9.648 S x l O 4 C/mol)(0.122 3 J/C)
= -23.60 kJ/mol
AS" = «£(3.06 x IO 5 v/K)
= (2)(9.648 5 x IO4 C/mol)(3.06 x 10"5 V/K)
= 5.90 C-V/(Kmol) = 5.90 J/(Kmol), where wc made use of the
conversion coulomb-volt = joule.
13-34.
In the right half-cell, the reaction Hg 2+ + Y4" ^ HgY2- is at equilibrium, even
though the net cell reaction Hg 2+ + H2 ^ Hg(/) + 2H + is not at equilibrium.
13-35.
(a)
AgC\(s) + e" ^
"
Ag(s) + CF
H + + e" T* \ H2(g)
AgCl(s) + ¿H 2 (g) ^
13-36.
EX = 0.222 V
£1 = 0 V
Ag(s) + H+ + CI"
£ = 0.222 - 0.059 16 l o g f H / ^
1
(b)
0.485 = 0.222-0.059 16 log '
1 ^
(a)
Left:
quinone + 2H + + 2c" ^
£* = 0.222 V
' => [CF] = 0.143 M
hydroquinone
Right: Hg 2 Cl 2 + 2e" ^ 2Hg(/) + 2CF
0.059 16, [hydroquinone]
-
£(left) = 0.700 - — j — log [ ¿ J ^ J
£(right) = 0 . 2 6 8 - Q Q 5 2 9 1 6 log[Clf
(b)
£(cell) = £(right) - £(left)
El = 0.700 V
EX = 0.268 V
184
Chapter 13
, { o , 6 8 _aop l o g t c | f } _ K « ,
« c e l l ) - - 0 432 - 2=21^16
M }
[quinonclfH+fíClT
v
b
'
2
[hydroquinone]
Setting [CF] = 0.50 M and noting [quinone] = [hydroquinone], we find
£(ccll) = -0.432 - 0.059 16 log (0.50) - 0.059 16 log [H+]
£(ccll) = -0.414 + 0.059 16 pH (A = -0.414, B = 0.059 16 V per pH unit )
(c) £(cell) = -0.414 + 0.05916(4.50) = -0.148
Since £ < 0, electrons flow from right to left (Hg —> Pt) through the meter.
13-37.
H+ (1.00 M) + e" == 5 H2 fe, 1.00 bar)
" H + ( J C M ) + c"
H + (1.00 M)
F*
?±
EX - 0 V
\ H2(g, 1.00 bar)
EL -
H + (JC M)
0 V
£" = 0 V
£ = 0.490 = 0 - 0,059 16 log [H+] => [H+] = 5.2 x 10<J M
Ab
13-38.
[RNH3+][OH-]
"
[RNH2]
(0.050)(AV[H+])
0.10
~
96
*
10
M 2+ + 2e" == M(s)
" 2H+ + 2e' ^
EX = -0.266 V
H2 (g, 0.50 bar)
El = 0 V
H2(g) + M 2 + =* 2H+ + M(J)
£* = -0.266 V
+ 2
£ =
**«
0-059 16,
fH l
- 0 . 2 6 6 - ^ — ^ ^ ^
[H+] in the left half-cell is found by considering the titration of 28.0 mL of the
tetraprotic pyrophosphoric acid (abbreviated H4P) with 72.0 mL of KOH.
28.0mLof0.0100MH 4 P = 0.280 mmol
72.0mLof0.0100MKOH - 0.720 mmol
First, 0.280 mmol OH" consumes 0.280 mmol of H4P, giving 0.280 mmol of H3Pand (0.720 - 0.280 =) 0.440 mmol of OH". Then 0.280 mmol OH- consumes 0.280
mmol of H3P-, giving 0.280 mmol of H2P2- and (0.440 - 0.280 =) 0.160 mmol of
OH-. Finally, 0.160 mmol of OIF reacts with 0.280 mmol of H2P2- to create 0.160
mmol of HP3-, leaving 0.120 mmol ofunreacted H2P2".
H4P
0.280 mmol
+
OH"
0.720 mmol
->
H2P2
0.120 mmol
+
HP30.160 mmol
pH = pA-3 + l o g j ^ ^ = 6.70 + log § ^ | § = 6.82 => [H+] = 1.50x 10-7 M
185
Fundamentals of Electrochemistry
Putting the known values of [H+] and PH2 i n t 0 t n e Nernst equation gives
0.05916 ,
fH+12
-0.246 = -0.266 - — j — log ^
^
.[M2+] = ,l3xio-,M
» ^ - ^ ^ S
In the right half-cell we have the equilibrium
.. ,
M2+
0.280
initial mmol/mL
"Too""
. .. ,
^
,
u
t
+
it
final mmol/mL
FEDTA
0.720
"ïQTJ"
O-
•*
MY2"
—
440
() 3SIJ
-
~foo-
small
100
. _
TMY2-]
0-280/100
.=
f
* " [M2+] a Y4 . FEDTA " (2-h -10'3)(0.004 2)(0.440/100)
13-39.
right half-cell: Pb2+(right) + 2e"== Pb(i)
2
left half-cell: ~ Pb +(left) + 2e' ^
2+
EX - -0.126 V
Pbjs)
EL = -0.126 V
2+
Pb (right) ^ Pb (left)
Nernst equation for net cell reaction:
005916,
rPb2+(left)1
rt™,c
x |()14
£* = 0
[Pb^(left)1
-0.0018 = - — j — t a g ¿ f r i g h t ) ] ^ [Pb^right)]
=
l l 5
For each half-cell, we can write [CO2' ] - Ksp (for PbC03) / [Pb2+]
[CQ23'(left)]
^sp(forPbCQ3y[Pb2+(left)] _ 1
[C023(right)] = i^sp (for PbC03)/[Pb2+(right)] " 1.15 "• "
In each compartment the Ca2+ concentration is equal to the total concentration of
all carbonate species (since PbC03 is much less soluble than CaC03). Let the
2-
•>
fraction of all carbonate species in the form C 0 3 be acó"}"
(i.e., [CO2"] = acó 2 ' [total carbonate]). We can say that [Ca2+] = [total carbonate]
= [CO2"] / a c o y The value of aco 2 " is the same in both compartments, since the
pH is the same. Now we can write
*SD(calcite) _ [Ca2+(left)][CQ23'(left)]
_ [CQ23 (left)]2 / aco 2 ;
Ksp(aragonite) " [ Ca ^ (right)] [C023 (right)] " [C023 (right)]2/accy2"
= (0.87)2 = 0.76.
186
Chapter 13
Cu 2+ + 2e" ^ Cu(s)
13-40.
EX = 0.339 V
" Ni 2+ + 2e" =* Ni(.v)
EL = -0.236 V
Cu 2+ + Ni(.s) =* Cu(s) + Ni 2+
E° = 0.575 V
The ionic strength of the right half-cell is 0.10 M and ycu2+ = 0.405.
The ionic strength of the left half-cell is 0.010 M and yN¡2+ = 0.675.
n<io
n<7(
0 059 16
(0.002 5)(0.675)
0.512 = 0 . 5 7 5 - ^ 2 — l o g ^ 2 * ] ( o . 4 o 5 )
PA
ta
=> [Cu2+] = 3.09 x 10-5
M
^sp = [Cu 2+ Jy Cu 2 + [I0 3 ] 2 y | 2 j3 ) =(3.09 x 10"5)(0.405X0.10)2(0.775)2 =7.5 x 10-«
13-41.
E" is the effective reduction potential for a half-reaction at pH 7, instead of pH 0.
Since living systems tend to have a pH much closer to 7 than to 0, £*' provides a
better indication of redox behavior in an organism.
AW
13-12.
M
(a)
Pc2H
v
nm
°05916 1
4
£ = 0.731-^r-log/ic2H2[H+]2
(b)
£ = 0.731 + 0.059 16 tag [H+] - *&&
l o g ^
This is E" when pH = 7
(c)
13-43
13-43.
E" = 0.731 + 0.059 16 log (IO"7«*) = 0.317 V
rHCN]2
lo
g - ° ' ° 529 1 6 k»
££ • £•
*/> (CN>2 [H+] 2
t H + ] FHCN
Substituting [HCN] = . H + - K into the Nernst equation gives
Wmñvn
£
"0'373-
00S916
2
1
l0
[H+]2 F
"™
8([H + ] + /Ca) />(cN)2[H+]2
2
£ = 0.373 + 0.05916 log ([H+] + * a ) - ^ f
1
P2
^ log j g g L
This is F'when pH = 7
Inserting /¡Ta = 6.2 x 10"'0 for HCN and [H+] = IO" 700 gives
E" = 0.373 + 0.059 16 log (IO' 7 0 0 +62 x 10 10 ) =
0.041 V.
Fundamentals of Electrochemistry
13-44.
H 2 C 2 0 4 + 2H + + 2e" F= 2HCO2H
„ A
0.059 16,
[HCO2H]2
£ = 0.204 - — — log [H2 C 2 0 4 ][H+]2
n
[H+] FHCO2H
But[HC0 2 H] =
[H^] 2
and [H 2 C 2 0 4 ] =
[ H + ] + Ki
FH 2 C 2 Q 4
[ H + ] 2 + K[[H+]
+ gg-
Putting these expressions into the Nernst equation gives
0 05916
[H+]2FHèu,n([H+]2 + ^ ' O T + ^ 2 )
£ = 0.204 - - 2 - log ( [ H + ] + ^
[H+]2 F H 2 C 2 0 4 [H+]2
R
0 05916
[ H + P + ZMIH
£ - 0.204 - ^ 2 — tag ( [ H + ] + ^ ) 2
1
]-
1
K\K2
[H+]2
2
fr
0.05916
HCQ2n
•'ogFH2c204
2
This is £*' when pH = 7
Putting in [H+] = 10-7<X>y[,Ka = 1.80x 10"4, Ki = 5.62x IO"2, and
K2 = 5.42 x IO-5 gives E" = -0.268 V.
13-45.
£ = £* -0.059 16 log
[H2Rcd"]
^HQx]
[H+] FHOX
But[HOx] = [H +] + A : a a n d [ H 2 R e < 1 ]
=
[H'] 2 FH2Red\B*? + [H+]Kl+K\K2
Putting these values into the Nernst equation gives
[H + ] 2 Fn2Red" ([H+] + Ka)
lo
g[H+]F H Ox([H + ] 2 + [H+]/C, + KyK2)
E = E° - 0.05916
[H+] ([H+] +/Ca)
Fll2Red"
E = ET - 0.05916 l o g [ H + ] 2 ; [ H + ] ^ + %jT - 0.059 16 l o g - p — .
E"
Since E" = 0.062 V, we find E° = -0.036 V.
13-46.
0.05916,
£ = F - ^"2
tag
[HNQ2]
[N03l[H+]3
[H+]FHN02
But [HN0 2 ] =
[H +]
+ ^a
and
^ 3
] =
FN
°3-
Putting these values into the Nernst equation gives
0.05916
1
0.05916
E
log
+ 2
= * - "~
'
([H+] + * a )[H ] "
2
F
HN0 2
« FNC-3
lo
188
Chapter 13
IT'
nAV
nr^n
0059 16,
£• = 0.433 = 0 . 9 4 0 — ^ — t a g
H 2 P0 4 + H + + 2e" ^
13-47.
1
(10,7 +
^)(10.7)2 ^
K&
HP023" + H 2 0
EX
HPOJj- + 2H+ + 2e~ =* HPO2,' + H 2 0
EL = -0.234 V
H2PO4 ?= HPOj- + H +
& =- ^
tag^a2(forH3P04)
=
= 7.2 * 10"4.
ET = EX- EL
Q05 916
log(6.34x
2
10-8) = -0.213 V
£+ = £ * - 0.234 V - -0.447 V
13-48.
(a)
A - 0.500 =
(1.12 x l04M->cm-l)[Ox](1.00cm) + (3.82x 103 M-lCm-<)[Rcd](1.00cm)
But [Ox] = 5.70 x 10-5 - [Red]. Combining these two equations gives
[Ox] - 3.82 x 10-5 M a n d r Rc{ j] = j gg x 10-5 M
(b)
(c)
[S"] - [Ox] = 3.82 x 10-5 M md rgj =
S + e" ^ S"
- Ox + c" ?= Red
Red + S ^ Ox + S'
£V = E" + £1' = -0.092 V
Red
= j gg „
10 -5 M,
£V = ?
£*_'=-0.128 V
[OxirS'l
E" = 0.059 16 log g g j g g j = 0.036 V
CHAPTER 14
ELECTRODES AND POTENTIOMETRY
14-1.
(a)
AgCl(s) + e" ^ Ag(i) + CI"
Hg2Cl2(s) + 2e" ^ 2Hg(0 + 2CF
(b) £ - E+ - E_= 0.241 - 0.197 = 0.044V
14-2.
(a) 0.326 V (b) 0.086 V (c) 0.019 V (d) -0.021 V (e) 0.021 V
14-3.
£ = £+-£_
£ = {0.771 - 0.05916
tagjj^J}
-(0.241) = 0.684 V
14-4.
£ = F - 0.059 16 log AQ\0.280 = 0.268-0.059 16 log J^ci- => J^ci- - 0.627
14-5.
For the saturated Ag-AgCl electrode, we can write: £ = £* - 0.059 16 log 3\c\-Putting in £ = 0.197 and £* = 0.222 V gives J4a- = 2.65. For the S.C.E., we
can write: £ = £* - 0.059 16 tag J\Q\- = 0.268 V - 0.059 16 log2.65
= 0.243 V.
14-6.
(a) Cu2+ + 2e- ^ Cu(s)
E° - 0.339 V
(b) £ + = 0 . 3 3 9 - 2 ^ f i á l o g ^ 2 T j = 0.309 V
(c)
£ = £ + - £ _ = 0.309 - 0.241 = 0.068 V
14-7.
A silver electrode serves as an indicator for Ag+ by virtue of the equilibrium
Ag+ + e- F* Ag(s) that occurs at its surface. If the solution is saturated with
silver halide, then [Ag+] is affected by changes in halide concentration.
Therefore, the electrode is also an indicator for halide.
14-8.
Vç = 20.0 mL. Ag+ + e" ^ Ag(s) => £+ = 0.799 - 0.059 16 log j^—:j
0.1 mL:
[Ag+] -
(¿öf) ( ° 0 5 0 0 M > ( H U )
= 00493 M
Fraction
Original
Dilution
remaining concentration factor
£ = £ + - £ _ = {0.799-0.059 16 log0^493} -0.241 = 0.481 V
189
190
Chapter 14
30.0 mL: This is 10.0 mL past Ke => [Br] = ( ^ | ) (0.025 0 M) - 0.006 25 M
[Ag+] - Ksp/[Br] = (5.0 x 10-'3)/0.00625 = 8.0 x 10"I > M
E = £ + - £ _ = {0.799-0.059 16 logg Q J ^ , , , } -0.241 =-0.039 V
14-9.
The reaction in the right half-cell is Hg2+ + 2e" =* Hg(/)
£ = £+-£_
-0.027 = 0 . 8 5 2 - ^ P t a g 7 J 7 ^ - (0.241)
=> [Hg2+] - 2.7 x 10-22 M.
The cell contains 5.00 mmol EDTA (in all forms) and 1.00 mmol Hg(II) in
100 mL. 1.00 mmol EDTA reacts with 1.00 mmol Hg(II), leaving 4.00 mmol
EDTA.
[HgY2]
[HgY2-]
=
** [Hg2+][Y4-] " [Hg2+]aY4-[EDTA]
(1.00 mmol,'100 mL)
_
K =
Af
22
"" (2.7 x 10- )(0.30)(4.00mmoyi00mL) " 3 l X l(fi
14-10.
(a) Fe3+ + e" ^ Fe2"1"
(b)
ET - 0.771 V
£=£+-£_
-0.126 =0.771 -0.059 16 log j j ^ J - 0.241 => j j j ^ J = 1.2 x IO"
/¡^FeEDTA") =
[FeEDTA"]
2
=
/^FeEDTA ") " [Fe3+][EDTA4]
=
14-11.
[FeEDTA-1 [Fe2+]
[FeEDTA2-]" [ÍV+]
=
:
[FeEDTA2"]
[Fe2+][EDTA4-]
(\.QQ* 1 0 ^
UoO* l O ^ 1 " 2
x ,0M
) =
6 x I0
'°
=-0.429-0.059 16 log " - f ^ J 2 - - 0.197
[Cu(CN)2]
Putting in £ = -0.440 V and [Cu(CN)2"] = 1.00 mM gives [CN"] - 0.847 mM.
E = E+-E_
Now wc use the pH to see how much HA reacted with KOH:
initial mmol
final mmol
HA +
10.0
10.0 -x
OH" -> A"
x
—
JC
+ H20
191
Electrodes and Polentioinetry
pH = pffa(HA) + log
j ^
9.954 = 9.50 +log ]0Q_X
tK0H] •
=> x = 7.4 0 mmolofOH"
7.4n mmol
25.0 mL • °- 2 9 *
M
14-12.
Junction potential arises because different ions diffuse at different rates across a
liquid junction, leading to a separation of charge. The resulting electric field
retards fast-moving ions and accelerates the slow-moving ions until a steady-state
junction potential is reached. This limits the accuracy of a Potentiometrie
measurement, because we do not know what part of a measured cell voltage is
due to the process of interest and what is due to the junction potential. The cell
in Figure 13-4 has no junction potential because there arc no liquid junctions.
14-13.
II ' has greater mobility than K+. The HCl side of the HCl | KCl junction will be
negative because H+ diffuses into the KCl region faster than K+ diffuses into the
HCl region. K+ has a greater mobility than Na+, so this junction has the opposite
sign. The HCl | KCl voltage is larger, because the difference in mobility between
H* and K+ is greater than the difference in mobility between K+ and Na+.
14-14.
Relative mobilities:
K+ -K 7.62
NO3 -» 7.40
5.19 «- Na +
7.91 <- C\~
Both the cation and anion diffusion cause negative charge to build up on the left.
14-15.
Velocity = mobility x field = (36.30 x 1 0 s m2/(s*V)) x (7 800 V/m) = 2.83 x
IO"3 m s-1 for H + and (7.40 x 10 8 )(7 800) = 5.77 x 10-4 m s"1 for NO3. To
cover 0.120 m will require (0.120 m)/(2.83 x 10-3 m s'1) = 42.4 s for H + and
(0.120)/(5.77 x IO-4) - 208 s for NO^.
14-16.
(a)
E° = 0.799 V => K = 100.799/0.05916 = 3 . 2 0 * 10«
(b)
K' - 10°801/0.05916 = 3. 4 6x 1013. K1K = 1.08. The increase is 8%.
(c)
K = I0 n | 0 0 / 0 °59I6 = 49.0.
K' - 100102/0-059 16 = 530
K'lK - 1.08. The change is still 8%.
192
Chapter 14
14-17.
Both half-cellreactionsare the same (AgCI + e" v^ Ag + CI") and the
concentration of CI" is the same on both sides. In principle, the voltage of the
cell would be 0 if there were no junction potential. The measured voltage can be
attributed to the junction potential. In practice, if both sides contained 0.1 M HCl
(or 0.1 M KCl), the two electrodes would probably produce a small voltage
because no two real cells arc identical. This voltage can be measured and
subtracted from the voltage measured with the HCl j KCl junction.
14-18.
(a)
E
J
In phase a, wc have 0.1 M H+ (u = 36.3 x 10-8 m2 s 1 V 1 ) and 0.1 M CI"
(u = 7.91 x io» m 2 s-i v-l). In phase ß, we have 0.1 M K+
(« = 7.62x 10-8 m2 s-1 V'1) and 0.1 M Cl" (« = 7.91 x 10-8m2s-l V*1).
Substituting into the Henderson equation gives
._ (36.3 x 10-8)r0-Q.n + (7.62* I0-8)[0.1-0]-(7.91 * 1Q-8)[Q.l - Q . n
" (36,3- 10-8)[0-0.1] + (7.62 * 10-8)[0.1-0] + (7.91 * 10«)[0.1 -0.1] *
005916lQ g P6-3x 10-8X0.1) + (7.91 * 1Q-8)(0.1) _
0.05916log ( 7. 6 2 X io-8)(0.1) +(7.91 x 10"8)(0.1) "
(b)
269 mV
I
KCl concentration (M)
(c)
[HCl]
10-4 M
10-3 M
.vMHCI|4MKCl
9.1 mV
4.6 mV
26.9 mV
3.6 mV
10- M
57.3 mV
3.0 mV
10-' M
93.6 mV
4.7 mV
2
14-19.
y M HCl | ImM KCl
Ideally, the electrode should be calibrated at 37° using two buffers bracketing the
pH of the blood. It would be reasonable to use the MOPSO and HEPES buffers
in Table 14-3 that are recommended for use with physiologic fluids. The pH of
these standards at 37'C is 6.695 and 7.370. The standards should be
193
Electrodes and Poteniiomctry
thcrmostatted to 37° during calibration, and the blood should also be at 37*
during the measurement.
14-20.
Uncertainty in pH of standard buffers, junction potential, junction potential drift,
sodium or acid errors at extreme pH values, equilibration time, hydration of
glass, temperature of measurement and calibration, and cleaning of electrode
14-21.
The error measured in the graph is -0.33 pH units. The electrode will indicate
11.00-0.33 = 10.67.
14-22.
Saturated potassium hydrogen tartrate and 0.05 m potassium hydrogen phthalate
14-23.
If the alkaline solution has a high concentration of Na4 (as in NaOH), the Na+
cation competes with H + for cation exchange sites on the glass surface. The glass
responds as if H+ were present, and the apparent pH is lower than the actual pH.
14-24.
The junction potential changes from -6.4 mV to -0.2 mV. A change of 6.4 - 0.2
= +6.2 mV appears to be a pH change of+6.2/59.16 = +0.10 pH units.
14-25.
(a)
(4.63)(59.16 mV) = 274 mV. The factor 59.16 mV is the value of
(ÄTTn 10)/£at298.15K.
(b)
At 310.15 K (37°C), (RT In 10)/£
= (8.3145 J mol"1 K-')(310.15 K)(ln 10)/(96485 CmoH) = 61.54 mV
(4.63) (61.54 mV) = 285 mV.
14-26.
pH of 0.025 m KH2PO4/0.025 m Na 2 HP0 4 at 20°C - 6.881
pH of 0.05 m potassium hydrogen phthalate at 20°C - 4.002
¿'unknown-£S1 = £S2~£S1
pHunknown-pHsi " pHS2~pHsi
£unknown-(-18.3mV)
(+146.3 mV)-(-18.3 mV)
pHunknown 6.881
=
4.002 6.881
= ~57l73
mV/
PH
umt
£unknown ~ (~18.3 mV)
pHunknown = " -57.17 3 mV/pH unit + 6 8 8 1 "" 5 " 6 8 6
Observed slope = 57.173 mV/pH unit
. , ,
/?rin 10
Theoretical slope = ~p~
[8.31447 VC/(molK)][293.15
K] In 10
ftA€Q1,„
9.648 53 xlO 4 C/mol
" - - 0 . 0 5 8 17 V
194
Chapter 14
P
14-27.
==
(a)
observed slope
theoretical slope
=
-57.173 m V
58.17mV
=
0983
There is negligible change in the concentrations of the buffer species when
we mix the acid H 2 P0 4 with its conjugate base, HPOj". The ionic strength
of 0.025 0 m KH 2 P0 4 (a 1:1 electrolyte) is 0.025 0 m. The ionic strength of
0.025 0 m Na 2 HP0 4 (a 2:1 electrolyte) is 0.075 0 m. The total ionic
strength is 0.100 m.
[H + ]YH+[HP0 2 -]YIIPO^
(b>
K l =
[H2P04]YH2P04
B u t K 2 - IO' 7 1 9 8 and [H + ]YH+ = 10'PH = IO- 6865
T.
VHPCJ
^2[H 2 P04]
lQ-7.198fo.Q25 Q]
2
therefore, ^ ^
- ^ ^ [ H P O - ] " i0-6.865[0.025 0]
f
=
04645
(We can use molality or any other units for concentrations because they
cancel in the numerator and denominator.)
(c) To get a pH of 7.000, we need to increase the concentration of base (HPO2,")
and decrease the concentration of acid (H2PO4). To maintain a constant
ionic strength, we must decrease KH2P04 three times as much as we
increase Na 2 HP0 4 , because Na2HP04 contributes three times as much as
KH 2 P0 4 to the ionic strength. So let's increase Na 2 HP0 4 by x and decrease
KH 2 P0 4 by 3*.
[H + ]YH + [HPQ 2 -]YHPQ^
Kl
"
[H2P04]yH2P04
=*
„V.7IQ8= 10-7000[O.Q25 0 + xl m 464
"
[0.025 0-3jt]
(°- 5) => x = 0.001 8 m.
10
The new concentrations should be Na 2 HP0 4 = 0.026 8 m and KH 2 P0 4 =
0.019 6 m.
14-28.
Analyte ions equilibrate with ion-exchange sites at the outer surface of the
ion-selective membrane. Diffusion of analyte ions out of the membrane creates a
slight charge imbalance (an electric potential difference) across the interface
between the membrane and the analyte solution. Changes in analyte ion
concentration in the solution change the potential difference across the outer
boundary of the ion-selective membrane.
195
Electrodes and Potcnliometry
A compound electrode contains a second chemically active membrane outside the
ion-selective membrane. The second membrane may be semipermeable and only
allow the species of interest to pass through. Alternatively, the second membrane
may contain a substance (such as an enzyme) that reacts with analyte to generate
the species to which the ion-selective membrane responds.
14-29.
The selectivity coefficient Kl% tells us the relative response of an ion-sclcctive
electrode to the ion of interest (A) and an interfering ion (X). The smaller
K^,
the more selective is the electrode (smaller response to the interfering ion).
14-30.
A mobile molecule dissolved in the membrane liquid phase binds tightly to the
ion of interest and weakly to interfering ions.
14-31.
A metal ion buffer maintains the desired (small) concentration of metal ion from
a large reservoir of metal complex (ML) and free ligand (L). If you just tried to
dissolve IO 8 M metal ion in most solutions or containers, the metal would
probably bind to the container wall or to an impurity in the solution and be lost.
14-32.
Electrodes respond to activity. If the ionic strength is constant, the activity
coefficient of analyte will be constant in all standards and unknowns. In this
case, the calibration curve can be written directly in terms of concentration.
14-33.
(a)
-0.230 = constant -0.059 16 tag (1.00 x 10'3) => constant - -0.407 V
(b)
-0.300 = -0.407-0.059 16 logjc => x = 1.55 x IO"2 M
(c)
-0.230 = constant - 0.059 16 log (1.00 x IO"3)
-0.300 = constant- 0.059 16 log x
subtract: 0.070 = -0.059 16 log - — j
14-34.
£i - constant + - ^
E2 = constant +
=> x = 1.52x 10"2M
log [1.00 x 10*4]
0.05916. rtnn
,„,.
^
log [1.00 x 10"3]
„
„
r,
0-05916. 1.00 x 10-3
A£ = £ 2 - £ . = — 2 — • < * 1.00*1 <H
14-35.
=
.___.._.
+ 0 0 2 9 6 V
[F-]Pr0Videncc = 1.00 mg F/L = 5.26 x 10-5 M
£providence = constant - 0.059 16 log [5.26 x 10'5]
£|;oxboro = constant - 0.059 16 log [F-]Foxboro
196
Chapter 14
À£ " £Foxboro - £providence = 0.040 OV
A nm
.^ i
I
JFoxboro
= -0.059 16 l o g 5 2 6 > <
14-36.
10.5
r„
.
,
=> [F-] F o x b o r o = 1.11 x 10-5 M = 0.211 mg/L
K+ has the largest selectivity coefficient of Group 1 ions and therefore interferes
the most. Sr 2+ and Ba 2+ are the worst of the Group 2 ions. Since log KPo! , =
1-1 ,K
+
+
-2, there must be 100 times more K than Li to give equal response.
14-37.
0.030 M
7 ^ ^ = 4.0x108 =
[M][L]
[MK0.020 M)
14-38.
(a)
[M] = 3.8 x 10-9 M
The least squares parameters arc
£ = 51.10 (±0.24) + 28.14 (±0.085) log [Ca2+]
(sy = 0.27)
40
-3
-2
Log [CaZ+l
14-39.
-1
0
(b)
The slope is 0.028 14 V = ß(0.059 16 W)¡2 => ß = 0.951.
(c)
If we use Equation 4-27 in a spreadsheet, we find log [Ca2+] =
-2.6153(±0.0072) using sy = 0.3 and * = 4.
From Table 3-1, we can write that if £ = 10*, e F /£ = (In \0)ex.
In this problem, £ = [Ca2+] = lO-2-°153(±0.0072)
ep/F = (In 10)(0.OO72) = 0.0165
ep = (0.016 6 )£ = 4.0 x 10-5 => p = 2.43Í+0.04) x 10-3 M .
At pH 7.2 the effect of H + will be negligible because [H+] « [Lt+]:
-0.333 V = constant + 0.059 16 log [3.44 x 10-4] => constant = -0.128 V.
At pH 1.1 ([H+] = 0.079 M), we must include interference by H + :
£ = -0.128 + 0.059 16 log [3.44 x l(H + (4x 10-4)(0.079)] = -0.331V.
197
Electrodes and Potentiomctry
14-40.
The function to plot on the v-axis is ( V0 + Fs) 10L'/S, where S = -(ß/fTln 1 Q)/nF.
(The minus sign comes from the equation for the response of the electrode, which
has a minus sign in front of the log term.) Putting in ß = 0.933, R = 8.314 5
J/(molK), £ = 9 6 485 C/mol, T= 303.15 K, and n = 2 gives 5 = -0.028 06 J/C =
0.028 06 V. (You can get the relation of J/C = V from the equation AC = -nFE,
in which the units are J = (mol)(C/mol)(V).)
F s (mL)
0
0.100
0.200
0.300
0.800
£(V)
0.079 0
0.072 4
0.065 3
0.058 8
0.050 9
y
0.084 1
0.144 9
0.259 9
0.443 8
0.856 5
The graph has a slope of m - 0.989 and an intercept of b = 0.080 9, giving an xintercept of-Mm - -0.081 8 mL. The concentration of original unknown is
(x-intercept) c s
ex = -•
(-0.081 8 mL)(0.020 0 M)
= 3.0 x IO"5 M.
55.0 mL
10-,
o 0.8 -
y = 0.989X + 0.0809
+ 0.6-
Intercept =
-0.081 8 mL
°- 2
J
-0.2
14 41.
(a)
0.0
0.2
0.4
0.6
V s (mL)
L
I
0.8
I
i
L
1.0
The following spreadsheet and graph are shown. The jc-intercept is at-3.65
mL with a standard deviation in cell B26 of s ~ 0.484 mL. The intercept
gives us the concentration of ammonia nitrogen in the volume F0 = 101.0
ml
3.65 mL= - cs
(x-intcrccpt)cs (3.65 mL)(10.0 ppm)
ex 101.0 mL
-0.3614 ppm
Vo
^-intercept
198
Chapter 1 4
A
B
C
1 Standard Addition: / ammonia in Seawater
2
3
V0 =
101 mL
4
Cs =
10 Ppm
5
6
7
e
g
10
n
12
13
14
15
1b
17
18
s =
D
E
F
G
H
0 0566 V
X
Added standard
E = cell
(mL)
voltage (V)
0.00
-0 0844
10.00
-0.0581
20.00
-0.0469
30.00
-0.0394
40.00
-0.0347
y
Vo + V. <Vo + V . ) 1 0 "
(mL)
101.0
111.0
121.0
131.0
141.0
(mL)
3.26
10.44
17.95
26.37
34.37
LINEST oulpul:
0.7815
2.8502 b
0.0137
0.3364 Sb
s™
R'
0.9991
0.4343
Highlight cells B17:D19
Type'=LINEST(D10:D14,A10:A14,TRUE,TRUE)
Press CTRL+SHIFT+ENTER (on PC)
m
19
Press COMMAND+RETURN (on Mac)
«»V
20
21 x-intercept = -tiim =
-3.647 mL
To find 95% confidence interval
22 n =
5 822 = COL)NT(A10:A14)
we need Student's t for
18.479 B23 = AVE RAGE(D10:D1 4 )
23 Mean y =
3 degrees of freedom
24 E(x - mean x)'' =
1000 B24 = DEVSQ(A10:A14)
TINV(0.05,3) = 3.182446
25 Std deviation of
t*81.541109
26 x-intercept =
0.484 mL
27 B26 = {C19/ABS(B17 ))*SQRT((1 /B22) + B2 3A2V(B17A2*B2<i»
I
y = 0.7815x +2.8502
>
+
Intercept = 15
-3.65 mL
>
-20
-10
0
10
20
V5 (volume of standard, mL)
30
I
199
Electrodes and Potcntiomclry
The concentration of ammonia nitrogen in the original 100.0 mL of
seawater, which had been diluted from 100.0 to 101.0 mL, is
loOOmL^0-3614 PP™) = °' 3 6 5 PPm- T h c 9 5 %
to Student's I times thc standard deviation:
c o n f i d e n c e i n t e r v a l is c m j a l
95% confidence interval - ±fs = ±(3.18)(0.484mL) = ±1.54 mL
where t is for 95% confidence and 5 - 2 = 3 degrees of freedom because
there are 5 data points on the standard addition curve. You can find t in the
table of Student's / or you can compute it with the statement
"=TINV(0.05,3)" in cell G24 in thc spreadsheet. The confidence interval of
±1.54 mL corresponds to a relative uncertainty of 100 x 3 65 m¿ = 42%.
Thc absolute uncertainty is (0.042)(0.365 ppm) = 0.15 ppm. The
concentration of ammonia nitrogen in the seawater can be expressed as 0.36
±0.15 ppm.
(b) Added standards should increase analytical signal by a factor of 1.5 to 3. In
this experiment, analytical signal is (VQ + Ks)10£/? = 3.26 mL for unknown
and 34.37 mL for final standard. The final signal is 10 times as great as the
initial signal, which is ~3 times more than the recommended limit,
14-42.
For the first line of data, with A = Na* and X = Mg2+
J IO'3 ^
*\(10"3),/2J
.
(+1X96 483 C/moIX 0.385 V)
" (8.3145 V-C/[mol-K])(294.65K) In 10
For thc second line of data, log K^ = -8.15.
=
Thcfirstand second lines should give the same selectivity coefficient. Thc
difference is experimental error.
For the third line of data, with A = Na' and X = K+:
, * *.
(+1X96 485 C/molX-0.285V)
J
10° ^ =
log A x
* (8.3145 V-C/[mol-K])(294.65 K)In 10 ' ^ ( Î O V ' J
For the fourth line of data, log K™x = -4.87.
14-43.
ForNa': error in JV(%) 2+
ForCa : error in J¡V(%) ~
vn0'
8
V(10'12 0 ) x
(IQ-«-»)'-' —
no'7Y'no"2-"j
,0
°
=
(H)-K<V' ' * 1 0 ° "
°- 2 5 %
25%
200
14^14.
Chapter 14
E = constant +
ß(
°°f
A
16)
tag ([Ca2+] + K™.
Mgï+
[Mg2+])
B
For the first two solutions we can write
-52.6 mV = A + B log (1.00 x i o 6 ) - A - 6 B
+ 16.1 mV = A + B log (2.43 x 1(H) = A - 3.614 B.
Subtraction gives 68.7 mV = 2.386 B => B = 28.80 mV.
Putting this value of B back into the first equation gives A = 120.2 mV.
The third set of data now gives the selectivity coefficient:
-38.0 mV = 120.2 + 28.80 log [10-* + Kv°\+ u (3.68 x io-3)]
Ca
*
O.M8-
«Mg
=6-0x10-4
E = 120.2 + 28.80 log ([Ca2+] + 6.0 x IO"4 [Mg2+]).
14-45.
There is a large excess of EDTA in the buffer. We expect essentially all lead to
be in the form PbY2- (where Y = EDTA).
[pbY21
=
T u f ö ( ° 1 0 M ) = 9.9 x 10-4 M
Total EDTA = { ^ (0.050 M) = 0.0495 M
Free EDTA - 0.0495 M - 9.9 x 10-4 M = 0.048s M
EDTA bound
toPb2*
2
Kf = ay4-K{ = (1.46 x 10-8)(10'8 0) - 1.46 x 1010
Pb 2+ + Y 4- =¿ PbY rPbY 2 ~l
W~ [Pb2+][EDTA]
fB,,..
[
14-46.
pb2+
rPbY 2 1
l « /vVÎËDTAÎ
9.9 * 10-4
=
(1.46x1010X0.0485)
=
M
* I0"¡2
M
[Hg2+] in the buffers is computed from equilibrium constants for the solubility of
HgX2 and formation of complex ions such as HgXj . Since the data for HgCFi
are not in line with the data for Hg(NC>3)2 and HgBr2, equilibrium constants used
for the I IgCl2 system could be in error. Whenever we make a buffer by mixing
calcidated quantities of reagents, we are at the mercy of the quality of tabulated
equilibrium constants.
201
Electrodes and Potentiometry
14-47.
(a)
slope = 29.58 mV =
log
^
E2-E1
"
2 _ ,og ^ ,
(-25.90)-2.06
l o g jzi 2 _ (-3,000)
=$ JA2 = 1.13 x 10-4
Ca 2+
(b)
5.00 x IO"4 -x
+
A3"
(0.998)1(5.00 K IO"4)-*]
^
CaA"
*
But Ji\ca2+ - 1.13 x IO"4 = (5.00 x 1 ( H - x ) (0.405)
Î
y from Table 7-1
=> x = 2.2 x IO"4 M
[CaA'JYCaA[Ca ]yca2+[A3-lYA3(2.20 x 10-4)(0,79)
Kf
4
~ (1.13 x 10- )[(0.998)(5 x 10-4-2.20 x 10^)](0.115)
K{ = 4.8 x IO4
f =
14-48.
2+
Analyte adsorbed on the surface of the gate changes thc electric potential of thc
gate. This, in turn, changes the current between the source and drain. The
potential that must be applied by the external circuit to restore the current to its
initial value is a measure of the change in gate potential. Following the Nernst
equation, there is close to a 59 mV change in gate potential for each factor-of-10
change in activity of univalent analyte at 25°C. The key to ion-spccific response
is to have a chemical on thc gate that selectively binds one analyte.
CHAPTER 15
REDOX TITRATIONS
15-1.
(a) Ce4+ + Fe2* -» Ce3+ + Fe3+
3+
(b) Fe + e"
Ce4+ •i e"
T^
V
a
Fe2+ ET = 0.767 V
Ce3+ ET = 1.70 V
(c) E= {0.767 - 0.059 16 log { j ^ } - {0.241}
(A)
E= {1.70-0.059 16 log j § ^ J } - { 0 . 2 4 1 }
(d)
15-2.
(a)
10.0 mL:
Use Eq. (A) with [Fe2+]/[Fe3+] = 40.0/10.0, since
yc - 50.0 mL => E = 0.490 V.
25.0 mL:
49.0 mL:
50.0 mL:
[Fe2+]/[Fe3+] = 25.0/25.0 => E = 0.526 V
[Fe2+]/[Fe3+] = 1.0/49.0 => E = 0.626 V
This is Ke, where [Ce3+] = [Fe3+] and [Ce4+] = [Fe2*].
Eq. 15-11 gives E+ = 1.23 V and E = 0.99 V.
51.0mL:
60.0 mL:
100.0 mL:
Use Eq. (B) with [Ce3+]/[Ce4+] = 50.0/1.0 => E = 1.36 V.
[Ce3+]/[Ce4+] = 50.0/10.0 => E = 1.42 V
[Ce3+]/[Ce4+] = 50.0/50.0 => E = 1.46 V
Ce4* + Cu+ -» Ce3* + Cu2+
(b) Ce4+ + e- =i Ce3+
Cu2+ + e~ =* Cu+
(c)
E=
E' = 1.70 V
E° = 0.161V
ÍCe3+li
{l.70-0.05916log|^|}-{0.197}
£ = {0.I6I-0.059 16
(d)
(B)
tagj^j}-
{(0.19?}
(A)
(B)
l.OOmL
Use Eq. (A) with [Ce3+]/[Ce4+] - 1.00/24.0, since
Ve = 25.0 mL => E = 1.58 V.
12.5 mL
24.5 mL
[Ce3+]/[Ce4+] = 12.5/12.5 => E = 1.50 V
[Ce3+]/[Ce4+] = 24.5/0.5 => E = 1.40 V
jCe3*!
E+ = 1.70 -0.059 16 log
[Ce4+]
25.0 mL
E+ = 0.161 -0.059 16 log -j~^:
2E+= 1.86, -0.059 16 log
202
g ^ f f j
203
Redox Titrations
At the equivalence point, [Ce3*] = [Cu2*] and [Ce4*] = [Cu*].
Therefore, the log term above is zero and £(- = 1.86|/2= 0.930 V.
E = 0.930-0.197 = 0.733 V
25.5 mL:
30.0 mL:
50.0 mL:
15-3.
Use Eq. (B) with [Cu*]/[Cu2*] = 0.5/25.0 => E = 0.065 V.
[Cu*]/[Cu2*] = 5.0/25.0 => /•: = 0.005 V
[Cu+]/[Cu2*] = 25.0/25.0 => E = -0.036 V
(a)
Sn 2+ + Tl 3 * -» Sn4* + Tl*
(b)
Sn 4 + + 2c" ^
Sn2* ET = 0.139 V
Tl 3 * + 2e" r* Tl* E' = 0.77 V
(c)
E = {o.l39-
005
£={0.77(d)
0.05916 ,
2
[Sn2+1
2 "«W-M-}
(A)
"Sog^}-{0.24,}
(B)
I.OOmL:
Use Eq. (A) with [Sn2*]/[Sn4+] = 4.00/1.00, since
Vt = 5.00 mL => E = -0.120 V.
2.50 mL:
[Sn2+]/[Sn4*] = 2.50/2.50 => E = -0.102 V
4.90 mL:
[Sn2*]/[Sn4+] - 0.10/4.90 => E - -0.052 V
0.05916, ISn^l
E+ ••- 0.1395
log[Sn4*]
2
0.05916
rn*i
log
E+ = 0 . 7 7 3
5.00 mL:
[Tl *]
0059 16,
[Sn2+lfTI+l
,0
2£+ = 0.90 9 ~
j
H '[Sn4+][T13 *]
rt™
At the equivalence point, [Sn4*] = [Tl*] and [Sn2*] - [Tl3*].
Therefore, the log term above is zero and £+ = 0.90o/2 = 0.454 V.
E = 0.45 4 -0.241 = 0.21 V
15-4.
5.10 mL:
Use Eq. (B) with [Tl*]/[T13+] = 5.00/0.10 => E = 0.48 V
10.0 mL:
Use Eq. (B) with [T1*]/[TI3*] = 5.00/5.00 => E = 0.53 V
(a)
2Fe3 * + ascorbic acid + H2O -» 2Fe2* + dehydroascorbic acid + 2H*
(b)
The equivalence volume is 10.0 mL.
At 5.0 mL, half of the Fe3* is titrated and the ratio [Fe2+]/[Fe3*] is 5.0/5.0:
Fe3+ + e" ^
Fe2*
ET = 0.767 V
E = E+-E- - {0.767-0.059 16 log j ^ j i j } - {o.l97>
204
Chapter 15
= {0.767 -0.059 16 logf$} - {0J97> = 0.570
10.0 mL is the equivalence point. We multiply the ascorbic acid Nernst
equation by 2 and add it to the iron Nernst equation:
TFe2*!
E+ = 0.767 - 0.059 16 log J j j ^ j
TIT
tin inn PJ)59_16
[ascorbic acid]
2£+ = 2 ^ 0 . 3 9 0 - ^ — log • d e M r o ] [ H + ] j
3£+ = 1.547 - 0.059 16 log
£t$T^™+,\
3
[Fe +][dehydro][H+]2
At the equivalence point, the stoichiometry of the titration
reaction tells us that [Fe2*] = 2[dehydroascorbic acid] and
[Fe3*] = 2[ascorbic acid]. Inserting these equalities into the
log term just shown gives
3E+ = 1.547 - 0.059 16tag9f ^rdehydroirascorbic acid]
e
2[ascorbic acid][dehydro][H+]2
3E+ = 1.547-0.059 16 log 7 ^ 2
Using [H+] - IO"030 gives £ + = 0.504 V and £ = 0.504 - 0.197 = 0.307 V.
At IS.OmL. the ratio [dehydro]/[ascorbic acid] is 10.0/5.0:
dehydroascorbic acid + 2H+ + 2e" == ascorbic acid + H2O F = 0.390 V
r
c nin ,ftA 005916, [ascorbic acid] > e
i
E = E+-E- - { 0 . 3 9 0 - - ^ l o g ' d e h y d r o ] [ H ^ } - {0.197}
f A . „ 005916.
[5.0]
> (
s
= { 0 . 3 9 0 - ^ 2 — 'og [100 j [10 .'o.3 0] 2? - {0.197} - 0.184 V
15-5.
(a) Titration reaction: Sn2+ + 2Fe3* -> Sn4+ + 2Fe2+
(b) Fe3* + e" =* Fe2*
ET = 0.732V
4
2
Sn * + 2e" F= Sn *
ET = 0.139 V
Fe = 25.0mL
(c) £ = {0.732 - 0.059 16logj^¿j} - {0.24l}
(A)
«-{"»-"P^gg}-{•«!}
(B)
(d)
Representative calculations:
in 1 r
«,,„
005916, [Sn42*l
LOmL: £+ = 0.139
^
'^[Sn *]
initial mol Sn2* = (25.0 mL)(0.050 0 n ^ ) = 1.25 mmol
mol Fe3* added = (1.0 mL)(0.100 ~ ^ - ) = 0.10 mmol
205
Redox Titrations
0.059 16 . [Sn42*]
£+
j
'^[Sn *]
0.05916. 4.62 x 1Q-2]
£ + = 0.139 - — 2 " " ' ° 8 [I.92X10-3]
. ,™
= 0.139
=
ftÄA_„
V
°098
£ = £ + - £ . = 0.098-0.241 = -0.143 V
25.0 mL: At the equivalence point, we add thc two indicator electrode
Nernst equations. To make the factor in front of the log term thc same in
both equations, wc can multiply the Sn4* | Sn2* equation by 2:
„ „Ä 0.05916, [Sn2*l
l0
£ + = 0.139 - — a
S [Sn4*]
[Sn2*]
2£+ = 0.278 - 0.059 16
tagb^j
[Fe2*]
£+ - 0.732- 0.059 16 log Jp^jj
Now add thc last two equations to get
3£+ = 1.010 - 0.059 16lQg(jSn4+j'[Fe3+jJ
But at thc equivalence point, 2[Sn4*] = [Fe2*] and 2[Sn2+] = [Fe3*].
Substituting these identities into the log term gives
, ÍISn2,12ÍSn4+fi
3£+ - 1.010 - 0.059 16 log ['[Sn4+j2[Sn2*]J
So the log quotient in the log term is I and the logarithm is 0. Therefore,
£+= 1.010/3 = 0.337 V and E = £ + - £ . - 0.337-0.241 = 0.096 V.
[Fe2*]
26.0 mL: £+ = 0.732- 0.059 16 log t ^ t
There is 1.0 mL of Fe3* beyond the equivalence point.
The first 25.0 mL of Fe3* were converted to Fe2*, so
[Fe2*]
£ + = 0.732- 0.059 16 log j ^ j
[4.90 x 10"21
£+ = 0.732- 0.059 16 log f ] 9ft x 10 - 3 j = 0.649 V
206
Chapter 15
£ = £ + - £ . = 0.649-0.241 = 0.408 V
mL
1.0
12.5
mL
£fV)
-0.143
-0.102
£(V).
24.0
-0.061
25.0
0.096
mL
26.0
30.0
£fV)
0.408
0.450
J
Volume of Fe3*
(mL)
15-6.
Diphenylamine sulfonic acid: colorless —• red-violet
Diphenylbenzidine sulfonic acid: colorless —• violet
iris (2,2'-bipyridine) iron: red —• pale blue
Ferroin: red —* pale blue
15-7.
The reduction potentials are
Sn4* + 2e" ^
Sn2*
£ = 0.139 V
Mn(EDTA)" + e" =* Mn(EDTA)2-
£° = 0.825 V
The end point will be between 0.139 and 0.825 V. Tris(2,2'-bipyridine) iron has
too high a reduction potential (1.120 V) to be useful for this titration.
15-8.
Preoxidation and prereduction refer to adjusting the oxidation state of analyte to a
suitable value for a titration. The preoxidation or prereduction agent must be
destroyed so it does not interfere with the titration by reacting with titrant.
15-9.
2S 2 0 2 8 + 2H 2 0
">.
4Ag2+ + 2H 2 0
2H 2 0 2
boiling
boiling
2
^ > 4S0 4 + 0 2 + 4H+
boiling
>
4Ag+ + 0 2 + 4H+
O2 + 2H 2 0
207
Redox Titrations
15-10.
A Jonesreductoris a column packed with zinc granules coated with zinc
amalgam. Prereduction is accomplished by passing analyte solution through thc
column.
15-11.
Cr3* and TiO2* would interfere if they were reduced to Cr2+ and Ti3+. In thc
Jones reductor, Zn is a strong enough reductant to react with Cr3* and TiO2*.
E° = -0.764 for the Zn2* | Zn couple
ET = -0.42 for the Cr3*| Cr2* couple
ET = 0.1 for the TiO2* | Ti3* couple
In the Waiden reductor, Ag is not strong enough toreduceCr3* and TiO2*:
EL = 0.222 for the AgCl | Ag couple
15-12.
A weighed amount of the solid mixture is added to a solution containing excess
standard Fc2* plus phosphoric acid. Each mol of (NH4)2S2C<8 oxidizes 2 mol of
Fe2* to Fe3*. Excess Fe2* is then titrated with standard KMn04 tofindout how
much Fe2* was consumed by the (NH4)2S2C<8. The phosphoric acid masks thc
yellow color of Fc3*, making the end point easier to see.
15-13.
(a)
Mn04 + 8H+ + 5e" ^
Mn2+ + 4H 2 0
(b) MnO; + 4H+ + 3e~# Mn02(s) + 2H 2 0
(c)
15-14.
Mn04 + e" =* Mn024"
3Mn04 + 5Mo3+ + 4H+ — 3Mn2+ + 5Mo02+ + 2H 2 0
(16.43-0.04) = 16.39mLof0.01033MKMnO4 = 0.169 3 mmol of MnO¡,
which will react with (5/3)(0.169 3) = 0.282 2 mmol of Mo3*.
[Mo3*] = 0.282 2 mmol/25.00 mL = 0.01129 M (= original [MoO2]).
15-15.
2Mn04 + 5H 2 0 2 + 6H* - • 2Mn2+ + 50 2 + 8H20
(27.66 - 0.04) - 27.62 mL of 0.021 23 M KMn04 = 0.58637 mmol of MnO*
which reacts with (5/2)(0.586 3?) = 1.465 9 mmol of H 2 0 2 , which came from
25.00 mL of diluted solution => [H202] = 1.465 9 mmol/25.00 mL =
0.05864 M in thc dilute solution. Thc original solution was ten times more
concentrated = 0.5864 M.
208
15-16.
Chapter 15
(a)
Schemel:
2 [8H+ + Mn04 + 5e" — Mn2+ + 4H 2 0]
+7
+2
5 [H2O2 -* O2 + 2e- + 2H*]
-1
0
6H* + 2Mn04 + 5H 2 0 2 -» 2Mn2* + 5 0 2 + 8H 2 0
Scheme 2:
2 [MnÛ4 -> Mn2+ + 2 0 2 + 3e"]
+7 -2
+2
0
3 [H 2 0 2 + 2H+ + 2e- — 2H 2 0]
-2
•1
6H* + 2MnO¡ + 3H 2 0 2 -> 2Mn2+ + 4 0 2 + 6H 2 0
(b)
1.023gNaBOy4fbO
153.86 g/mol
= 6 6 4 9 mmo1 N a ß
03
One tenth of this quantity was titrated • 0.664 9 mmol NaB03, producing
0.664 9 mmol H 2 0 2 by the reaction BO3 + 2H 2 0 -* H2O2 + H2BO3.
In Scheme 1, 2MnO¡ react with 5H2O2
=> 0.664 9 mmol H 2 0 2 requires 5 (0.664 9) = 0.266 0 mmol MnO^
0.266 0 mmol MnO¡
0.01046mmol KMn04/ml
= 2543 m L K M n
°
4
squired
In Scheme 2, 2MnO^ react with 3H2O2
=> 0.664 9 mmol H2O2 requires § (0.664 9) = 0.443 3 mmol Mn04
0.443 3 mmol MnO^
0.010 46 mmol KMnOVml
15-17.
= 42M
mL KMn0
4 required
2MnO¿ + 5H 2 C 2 0 4 + 6H* - • 2Mn2+ + 10CO2 + 8H 2 0
18.04 mL of 0.006363 M KMn0 4 - 0.1148 mmol of MnO¡, which reacts with
(5/2)(0.1148) - 0.2870 mmol of H 2 C 2 0 4 , which came from (2/3X0.287 0) =
0.191 3 mmol of La3*.
15-18.
C3H8O3
+
glycerol
(average oxidation
number of C = -2/3)
[La3+] = 0.191 3 mmoi;50.00 mL = 3.826 mM.
3H 2 0
^
3HC0 2 H
+
8e" + 8H*
formic acid
(oxidation
number of C = +2)
8Ce4* + 8e" ^ 8Cc3+
C 3 H 8 0 3 + 8Ce4* + 3H 2 0 =* 3HC0 2 H + 8Ce3* + 8H+
209
Redox Titrations
One mole of glycerol requires eight moles of Ce4*.
50.0mLof0.083 7MCe 4 + = 4.185 mmol
12.11 mL of 0.044 8 M Fe2* = 0.543 mmol
Ce 4 * reacting with glycerol - 3.642 mmol
glycerol = (1/8) (3.642) = 0.4552 mmol = 41.9 mg => original solution •
41.9 wt% glycerol
15-19.
50.00mLof0.118 6MCe 4 + = 5.930 mmol Ce 4 *
3l.l3mLof0.042 89MFe 2 + = 1335 mmol Fe 2 *
4.595 mmol Ce 4 * consumed by N 0 2
Since two moles of Ce 4 * react with one mole of N 0 2 , there must have been
1/2(4.595) = 2.298 mmol of NaN0 2 = 0.1585 g in 25.0 mL. In 500.0 mL,
there would be ( ^ ) o . 158 5) = 3.170g = 78.67% of the 4.030 g sample.
15-20.
Step 2 gives the total Cr content of the crystal, since each Cr*+ ion ir
oxidation state is oxidized and reacts with 3Fe2*.
(0.703 mLÏ2.786 mM) _ 12.51 umol Fe2*
Ste 2:
P
0.156 6 g of crystal
g of crystal
y(12.51)pmolCr
g of crystal
4J69umolCr
g of crystal
Step 1 tells us how much Cr** is oxidized above the +3 state. Eacr
with ( x - 3) Fe2*.
(0.498 mLK2.786 mM)
Step 1: 0 437 5 g 0 f crystal
=
3.171 umol Fe 2 *
g of crystal
Since one gram of crystal contains 4.169 pmol of Cr that reacts with 3.171 pm of
Fe2*, the average oxidation state of Cr is 3 + ^J^ = +3.761.
Total Cr (from Step 2) = 4.169 pmol Cr per gram = 217 pg per gram of crystal.
15-21.
(a) Theoretical molarity = (3.214 g/L)/(158.034 g/mol) = 0.02033 7 M.
(b) 25.00mLof0.020 33 7 MKMnO 4 = 0.508 43 mmol. But two moles of Mn0 4
5
react with five moles of H3ASO3, which comes from 4 moles of As 4 0 6 .
The moles of As 4 0 6 needed to react with 0.508 4 3 mmol of Mn0 4 =
(l/2)(5/4)(0.50843) = 0.317 77 mmol = 0.125 74 g of As406-
210
Chapter 15
0.508 43 mmol KMnOd
u
0.125 7 4 g As 2 0 3
x mmol KMn0 4
=
0.146 8gAs 4 O 6 =* *
= 05936
'
mmo1
KMn0 4 in (29.98-0.03) = 29.95 mL =* [KMn0 4 ] = 0.01982 M.
15-22.
r reacts with I 2 to give I¡. This reaction increases the solubility of I 2 and
decreases its volatility.
15-23.
Standard triiodidc can be prepared from a weighed amount of KIO3 with acid plus
excess iodide. Alternatively, triiodide solution can be standardized by reaction
with standard S2O3* prepared from anhydrous Na 2 S 2 0 3 .
15-24.
Starch is not added until just before the end point in iodometry, so it does not
irreversibly bind to I2 which is present during the whole titration.
15-25.
(a) 50.00 mL contains exactly 1/10 of the KIO3 = 0.1022 g = 0.477 5 7 mmol
KIO3. Each mol of iodate makes 3 mol of triiodide, so I3 - 3(0.477 57)
= I.4327 mmol.
(b) Two moles ofthiosulfate react with one mole of I3. Therefore, there must
have been 2(1.43 27) = 2.86 5 4 mmol ofthiosulfate in 37.66 mL, so the
concentration is (2.865 4 mmol)/(37.66 mL) = 0.07608 7 M.
(c) 50.00 mL of KIO3 make 1.4327 mmol Ç, The unreacted l¡ requires 14.22
mL of sodium thiosulfate = (14.22 mL)(0.076087 M) = 1.0820 mmol, which
reacts with | ( 1.082o mmol) - 0.54In mmol I3. The ascorbic acid must have
consumed the difference = 1.4327 - 0.541 0 = 0.8917 mmol I3. Each mole of
ascorbic acid consumes one mole of Ç, so mol ascorbic acid = 0.8917 mmol,
which has a mass of (0.8917 x 10 3 mol)(176.13 g/mol) = 0.157, g. Ascorbic
acid in thc unknown = 100 x (0.157| g)/(1.223 g) = 12.8 wt%.
(d) Starch should not be added until just before the end point because I3 is
present throughout the titration and will irreversibly bind to starch if the
starch is added too early.
15-26.
2Cu2* + 51- -» 2GH(,) +1¡
ç
+ 2 S
^- _
3I- +
5^2-
23.33mLof0.04668MNa 2 S 2 O 3 = 1.0890mmol S 2 0^' = 0.5445 mmollj,
which came from 1.0890 mmol Cu 2 * - 69.20 mg Cu. This much Cu comes from
211
Redox Titrations
1/5 of the original solid, which therefore contained 346.0 mg Cu = 11.43 wt%.
There is a great deal of I3 present at the start of thc titration, so starch should not
be added until just before the end point.
15-27.
l^ + 2S2023" -+ 3 r + S 4 0 2 "
H2S + 1 3 ^ S(.ï) + 31- + 211+
25.00 mL of 0.01044 M I3 = 0.261 0 0 mmol I3
14.44 m L of 0.009 336 M Na 2 S 2 0 3 - 0.1348 ( mmol Na 2 S 2 03, which would
have reacted with 0.067 40$ mmol I3. Therefore, the quantity of I3 that reacted
with H2S was 0.261 0 0 - 0.067 406 = 0.193 5o mmol. Since 1 mol of I3 reacts
with 1 mol of H 2 S, thc H2S concentration was 0.193 5o mmoi725.00 mL =
0.007 744 M. I3 is present at the start of the titration, so starch should not be
added until just before the end point.
15-28. (a) \2{aq) + 2e" == 21"
31" r*
£* = 0.620 V
l3 + 2e-
g—0.535 V
£° = 0.085 V
\2(aq) + T r= I5
= 1 n2(0.085V0.059 16 = 7
K
(b)
x
I2(v) + 2e~ * - 21"
31" ÇÈ I3 + 2e"
l::(v)
+ r
=i
I3
IO 2
ET = 0.535 V
ET = -0.535 V
/•;
0.000 v
2
K - io (-° OO0)/0.059 16 =- 1.0
(c)
h(s) + 2c" ^
21"
ET = 0.535 V
ET = -0.620 V
IT ? i \2{aq) + 2e"
\2{s) ^ \2{aq)
E- = -0085 V
2
085
K ••• [h(aq)] = 10 (-° VO.°5916 = 1.3 x 10"3 M = 0.34gofI 2 /L
15-29.
Each mole of NH3 liberated in the Kjcldahl digestion reacts with 1 mole of H*
in the standard H 2 S0 4 solution. Six moles of H* left (3 moles of H 2 S0 4 ) after
reaction with NI I3 will react with I mole of iodate by Reaction 15-18 to release 3
moles of I3. Two moles ofthiosulfate react with 1 mole of I3 in Reaction
15-19. Therefore each mole ofthiosulfate corresponds to 2 mol of residual
H 2 S0 4 .
mol NH3 = 2 (initial mol H 2 S0 4 - final mol H 2 S0 4 )
212
Chapter 15
mol NH3 = 2 (initial mol H2S0 4 15-30.
2
x mol thiosulfate)
(a) IO3 + 81" + 6H+ =* 3I3" + 3H 2 0. The stock solution contained
{0.804 3 g KIO3 (FM 214.00) + 5 g KI (FM 166.00)} / 100 mL, which
translates into 0.037 58 M KIÛ3 plus 0.30 M Kl, giving a mole ratio
KI/KIO3 = 18, which is a good excess of the 8:1 ratio required in the
reaction. 5.00 mL of the stock solution contain 0.187 9 2 mmol KIO3 plus
1.5 mmol Kl. 1.0 mL of 6.0 M H 2 S0 4 contains 6 mmol H 2 S0 4 , which is a
large excess for the reaction. Neither Kl nor H 2 S0 4 needs to be measured
accurately.
(b) 15 + SO?j" + H 2 0 - • 31- + SOj" + 2H*
(c) 0.187 9 2 mmol KIO3 delivered to the wine generates 3 x 0.187 9 2 = 0.563 76
mmol I3. The excess, unreacted I3 required 12.86 mL of 0.048 18 M
Na 2 S 2 03 = 0.619 5ç mmol Na2S203. Each mole of unreacted I3 requires 2
moles of Na 2 S 2 03, so there must have been (0.619 5o)/2 = 0.309 8 mmol I3
left over from the reaction with sulfite. Therefore, the I3 that reacted with
sulfite was (0.563 7 6 - 0.309 8) = 0.254 0 mmol I3. One mole of I3 reacts
with 1 mole of sulfite, so there must have been 0.254 0 mmol SO2," in 50.0
mL of wine. [SOf] = 0.254 0 mmol/50.0 mL = 5.07 9 x IO*3 M. With a
formula mass of 80.06 for sulfite, the sulfite content is 406.6 mg/L.
w> • » - - V 2 - 2 2 ^ ; " 2 ' 3 " " - 2.-5
'calculated ~~
'table
=
1277.7 - 273.2] / 3 - 3
2 15
*\/3 + 3
—
2.56
2.776 for 95% confidence and 3 + 3 - 2 = 4 degrees of freedom
'calculated < 'table, so the difference is not significant at 95% confidence level.
15-31.
25.00 mL of 0.02000 M KB1O3 = 0.500 0 mmol of BrO^, which generates 1.500
mmol of Br2. One mole of excess Br2 generates one mole of I2 (from I") and one
mole ofI 2 consumes 2 moles of S 2 0 3". Since mmol of S2O3" = (8.83)(0.051 13)
= 0.451 5 mmol, I 2 = 0.225 7 mmol and Br2 consumed by reaction with
8-hydroxyquinoline = 1.500-0.225 7 = 1.274 mmol. But one mole of
213
Redox Titrations
8-hydroxyquinoline consumes 2 moles of Br2, so 8-hydroxyquinoline = 0.637 1
mmol and Al3* = 0.6371/3 = 0.2124 mmol - 5.730 mg.
15-32.
(a) YBa2Cu307 contains I Cu3* and 2 Cu2*. YBa2Cu306.5 contains no Cu3*
and 3 Cu2*. Thc moles of Cu3* in the formula YBa2Cu307-z are therefore
I -2z. The moles of superconductor in 1 g of superconductor are
(I g)/[(666.246 - 15.9994 z)g/mol]. Thc difference between experiments B
and A is 5.68 -4.55 = 1.13 mmol S20237g of superconductor. Since 1 mol
ofthiosulfate is equivalent to 1 mol of Cu3*, there are 1.13 mmol Cu3+/g of
superconductor.
mol Cu3*
1.13 10-3molCu3*
1fisuperconductor
]
mol superconductor = I - 2z = ~(
1(666.246 - 15.9994 z) g/molj
Solving this equation gives z = 0.125. The formula is YBa2Cu306.875(D;
I — ¿z —
1 -2z =
r5.68(±0.05)-4.55(+0.10)l IO'3
/
\
V666.246- 15.9994 z)
1.13 (±0.112) 10-3
1,666.246- 15.999 4 z)
1 - 2z = 0.752 86 (±0.074 49) - 0.018 079 (±0.001 789) z
0.247 124 (±0.074488) = 1.981 92 (±0.001 79) z
z = 0.125 ±0.038.
The formula is YBa2Cu306,875 ±0.03815-33.
A superconductor containing unknown quantities of Cu(I), Cu(II), Cu(III), and
peroxide (0?>") is dissolved in a known excess of Cu(I) in oxygen-free HCl
solution. Possible reactions are
Cu3* + Cu+ -> 2Cu2+
H 2 0 2 + 2Cu++ 2H+ — 2H 2 0 + 2Cu2+
Unreacted Cu(I) is then measured by coulometry tofindout how much Cu(I) was
consumed by the dissolving superconductor. The amount of Cu(l) consumed is
equal to the moles of Cu3* plus 2 times the moles of 022 in the superconductor.
The coulometry is done under Ar to prevent oxidation of Cu(I) by 0 2 from the air.
If the superconductor contained Cu(I) (but no Cu(III) or peroxide), then thc
amount of Cu(I) found by coulometry would be greater than thc known amount
used in the original solution.
214
15-34.
Chapter 15
(a) Initial Fe 2+ in 5.000 mL = (5.000 mL)(0.100 0 M) = 0.500 0 mmol.
K 2 Cr 2 07 required for titration of unreacted Fe 2*
= (3.22 8mL)(0.0l5 93MK 2 Cr2O 7 ) = 0.0514 2 mmol.
But I mmol K 2 Cr 2 07 reacts with 6 mmol Fe2* by thc reaction
K 2 Cr 2 07 + 6Fe2+ + 14H+ -> 2Cr3+ + 6 Fe 3* + 2K+ + 14H 2 0.
Therefore, Fe2* left after reaction with Li i + y Co0 2
= (0.0514 2 mmol)(6 mmol Fc2+/mmol K 2 Cr 2 0 7 ) = 0.308 5 mmol.
Fe 2 * consumed by Co 3 * • (0.500 0-0.308 5) = 0.191 5 mmol.
1 mol Fe 2+ is consumed by 1 mol Co3 h, so Co 3+ in 25.00 mg solid sample 0.191 5 mmol.
(b) Co in 25.00 mg solid - (0.564 g Co/g solid)(25.00 g solid) = 14.10 mg
Co in 25.00 mg solid = (14.10 mg)/(58.933 g/mol) = 0.239 3 mmol
From (a), we know that Co3* = 0.191 5 mmol, so
Co 2 * = 0.2393-0.191 5 = 0.047 8 mmol.
Co oxidation state = (0047 8 mmol)(2+) + (0.19l 5 mmol)(3+) _
L.O oxidation state
_ 2.80
Q m 2 mmol
(c) If Co has an average oxidation number of+2.80 and O has an oxidation
number of-2, Li must contribute a charge of 4 - 2.80 - 1.20. Therefore, the
formula is Li1.20.CoO2 and>> = 0.20.
(d) Theoretical weight percent for metals in Li|, 2 oCo0 2 :
Formula m a s s - 1.20(6.941)+ 1(58.933)+ 2(15.9994) = 99.261
wt%Li = 100 x 1.20(6.941)/99.261 = 8.39%
wi%Co = 100x58.933/99.261 - 59.37%
wt% Li
„
= 0 1 4 1 3
wl%Co"
The observed quotient wt% Li/wt% Co is 0.138 8 ±. 0.000 6, which is not
exactly equal to the quotient computed from thc oxidation number. The
difference represents experimental error between thc two methods used find
the stoichiometry.
15-35.
Denote the average oxidation number of Bi as 3 + b and thc average oxidation
number of Cu as 2 + c.
Bi^Sr^Ca^Cu^O,
Positive charge = 6 + 2b + 4 + 2 + 4 + 2c = 16+ 26 +2c
The charge must be balanced by O2" => x = 8 + b + c.
The formula mass of thc superconductor is 760.37 + 15.999 4(8 + b + c).
215
Redox Titrations
One gram contains 1/F760.37 + 15.9994(8 + b + c)] moles.
(a)
Experiment A: Initial Cu* = 0.2000 mmol; final Cu* = 0.108 5 mmol.
Therefore, 102.3 mg of superconductor consumed 0.091 5 mmol Cu*.
2 x mmol Bi 5 * + mmol Cu3* in 102.3 mg of superconductor = 0.091 5.
Experiment B: Initial Fe2* = 0.1000 mmol; final Fe2* - 0.0577
mmol. Therefore, 94.6 mg of superconductor consumed 0.042 3 mmol
Fe2*.
2 x mmol Bi 5 * in 94.6 mg of superconductor » 0.042 3.
Normalizing to 1 gram of superconductor gives
Expt A: 2(mmol Bi5*) + mmol Cu 3 * in 1 g of superconductor = 0.89443
ExptB: 2(mmol Bi5*) in 1 g of superconductor = 0.447 15
It is easier not to get lost in the arithmetic if we suppose that the oxidized
bismuth is Bi 4 * and equate one mole of Bi 5 * to two moles of Bi4*.
Therefore, we can rewrite the two previous equations as
mmol Bi 4 * + mmol Cu3* in 1 g of superconductor = 0.89443 (1)
mmol Bi 4 * in 1 g of superconductor • 0.447 15
(2)
Subtracting (2) from (1) gives
mmol Cu 3 * in 1 g of superconductor • 0.44728
(3)
Equations (2) and (3) tell us that the stoichiometric relationship in the
formula of the superconductor is b/c = 0.44715/0.447 28 = 0.9997.
Since 1 g of superconductor contains 0.447 28 mmol Cu 3+ , we can say
mol Cu3*
„
= 2c
mol solid
3
*
/
2c (8 + b + c)
mol
Cu
gram solid / mol
mol solid
solid " 760.37 + 15.9994
™o1 C" 3 *
¿ç
= 4 4728 x IO-4
(4)
w
gram solid " 760.37+15.9994(8 + 6 + c) 4 - 4 W * l u ^
Substituting b = 0.999 7c in the denominator of (4) allows us to solve for c:
760.37 + 15.9994(8 + 1.9997c)
=> b = 0.999 7c = O.2OO0
= 4.472 8 x io- 4 => c = O.2OO1
The average oxidation numbers are Bi3-2000* and Cu2 2 0 0 1 * and the formula
of thc compound is Bi2Sr2CaC11.2O8.40Op since the oxygen stoichiometry
derived at the beginning of the solution is x = 8 + b + c.
(b)
Propagation of error:
Expt A: 102.3 (±0.2) mg compound consumed 0.091 5 (±0.0007) mmol Cu*
Expt B: 94.6 (±0.2) mg compound consumed 0.042 3 (±0.000 7) mmol Fe 2 *
216
Chapter 15
Normalizing to 1 gram of superconductor gives
Expt A: mmol Bi4* + mmol Cu3* in 1 g of superconductor
0.091 5 (±0.000 7)
mmol
89443
0 0 7 0 6
• 0.1023 (+0.0002) • O '
^O)^
Expt B: mmol Bi4* in 1 g of superconductor
0.042 3 (+0.0007)
mmol
Ä a
• 0.0946 (±0.0002) = 0 4 4 7 1 5 <=«>•«" ^
^
3
mmol Cu *
g superconductor = 0.89443 (±0.007 06)-0.447 15 (±0.00746)
- 0.447 28 (±0.01027)
b
0.447 15 (±0.00746)
c = 0.44728 (±0.01027) " 0.999 7 (±0.028 4)
2c
760.37+ 15.9994(8+ [1.999 7(±0.0284)]c) ~ 4.472 8 (±0.102 7) x 10-4
[4471.47 (±102.7)] c = 888.365 + [31.9994 (±0.445)] c
=> c - 0.200 1 (±0.004 6)
The relative uncertainty in A just given as 0.00746/0.447 15 is smaller than
the relative uncertainty in c, which is 0.01027/0.44728.
. ,
0.00746/0.447 15
Uncertainty m b - Q 01027/0 447 28 ( u n c e r t a i n t y m c )
0.00746/0.447 15
= 0.01027/0.44728 ^ 0 0 0 4 6 ) " ± 0 0 0 3 3
=> b = 0.2000 (±0.003 3)
The average oxidation numbers are Bi*3 2 0 n 0(±0.003 3) a n d
Cu+2.2001 (±0.004
and thc formula of the compound is Bi2Sr2CaCu2O8,400 i(±o.005 7)-
6)
CHAPTER 16
ELECTROANALYTICAL TECHNIQUES
16-1.
We observe that the silver electrode requires -0.5 V more negative potential than
the platinum electrode for reduction of H3O+ to H2. The extra voltage needed to
liberate H 2 at the silver surface is the overpotential required to overcome the
activation energy for the reaction. In Table 16-1, wc see that the difference in
overpotential between Pt and Ag is -0.5 V for a current density of 100 A/m2.
16-2.
(0.100 mol)(96 485 C/mol) = 9.648 x 103 C
(9.648 x liPCVi 1.00 C/s) = 9.648 x 10 3 s - 2.68 h
16-3.
ET = -AGLI2F = -237.13 x l03/[(2)(964 85)] = -1.228 8 V
"Standard" means that reactants and products are in their standard states (1 bar for
gases, pure liquid for water, unit activity for H* and Ü11).
16-4.
(a)
£ = £(cathode) - £(anode)
= JF(cathode) - 0.059 1 6 1 o g P ^ [0 H"]j
- {F(anodc) - 0.059 16 log [Br"]}
(remember to write both reactions as reductions)
= {-0.828 - 0.059 161og(1.0) l/2 [0.l0]}
- {1.078 - 0.059 16 log [0.10]} = -1.906 V
(b)
Ohmic potential = PR = (0.100 A)(2.0 £2) = 0.20 V
(c)
£ • £(cathode) - £(anode) - IR - Overpotential s
= -1.906 - 0.20 -(0.20 + 0.40) = -2.71 V
(d)
£(cathode) = F(cathode) - 0.059 16 log p\Q [OH"]s
= -0.828 - 0.059 16 log (LO)" 2 [1.0] = -0.828 V
£(anode) = £*(anodc) - 0.059 16log[Br'] s
= 1.078 - 0.059 16 log [0.010] = 1.196 V
E = E(cathode) - E(anode) - I-R - Overpotentials
= -0.828 - 1.196 - 0.20 -(0.20 + 0.40) = -2.82 V
16-5.
V 2 is thc voltage between thc working and reference electrodes, which is held
constant. Working:
16-6.
(a)
O
Reference:
•
Auxiliary:
1
For every mole of Hg produced, one mole of electrons flows.
1.00 mL Hg = 13.53 g Hg = 0.067 45 mol Hg = 0.067 45 mol e".
217
218
Chapter 16
(0.067 45 mol) (96 485 C/mol) = 6 508 C.
Work = q-E = (6 508 C) (1.02 V) = 6.64 x io 3 J.
(b)
The power is 0.209 J/min = 0.003 48 J/s.
P = fiR => / = yfp/R = V(0.003 48W)/(I00Q) = 5.902 mA.
In 1 h thc total charge flowing through thc circuit is
(5.902 x io- 3 C/s)- (3 600 s) = 21.25 C/(96 485 C/mol)
= 2.202 x 10- 4 molofe7h = 1.101 x lO^mol ofCd/h
= 0.0l2 4gCd/h.
16-7.
Hydroxide generated at the cathode and CI" in thc anode compartment cannot
cross the membrane. Na+ from seawater crosses from thc anode to thc cathode to
preserve charge balance. Therefore, NaOH can be formed free from CI".
16-8.
£ = £(cathode) - £(anode) - IR - overpotcntials
Suppose that the open-circuit voltage of each cell is £(cathode) - £(anode) =
2.2 V when no current flows. Ohmic loss and overpotcntials for the two halfreactions decrease the output of the cell by 0.2 V, giving a net cell voltage of
2.0 V when the cell is delivering current. The cell can be recharged at very low
current flow by applying just over 2.2 V in the opposite direction to reverse the
cell chemistry. To charge at a significant rate requires additional voltage to
overcome ohmic loss and overpotcntials. The recharge requires -0.2 V more than
open-circuit voltage, or -2.4 V. Electrical losses [IR, ovcrpotentials, and
concentration polarization in the terms £(cathode) and £(anode)] always decrease
thc magnitude of thc voltage that can be delivered by a cell and increase the
magnitude of the voltage required to reverse the spontaneous cell reaction.
16-9.
Pb(lactate)2 + 2H 2 0 -* Pb0 2 (j) + 2 lactate" + 4H+ + 2e"
Pb 2 +
Pb 4+
Lead is oxidized to Pb0 2 at the anode.
Thc mass of lead lactate (FM 385.3) giving 0.111 1 g of Pb0 2
(FM = 239.2) is (385.3/239.2)(0.111 1 g) = 0.179 0g.
0 179 0
%Pb =- , / ^ g X 100 = 54.77%
Electroanalytical Techniques
16-10.
219
Cathode: Sn2* + 2e" ^
Sn(s)
£* = -0.141 V
£(cathodc, vs S.H.E.) = -0.141 - ° ^ y - ^ l o g
10xl0
. 8 = "0-378 V
£(cathode, vs S.C.E.) - -0.378 - 0.241 - -0.619 V
The voltage will be more negative if concentration polarization occurs.
Concentration polarization means that [Sn 2 *] s < 1.0 x IO 8 M.
16-11.
When 99.99% of Cd(II) is reduced, the formal concentration will be 1.0 x 10 5 M,
2+
and thc predominant form is Cd(NH3) 4 .
_ [Cd(NH3)24+] _ (LO xlO- 5 )
P 4 - [Cd2<][NH3]4 " [Cd2*](1.0)4 =*
Cd2* + 2c" F* Cd(.v)
£(cathode) = -0.402
16-12.
_
[Cd
]
2 8
lü
M
ET = -0.402
0.059 16.
1
n„***,
¿
log 7 ^ 7 ] = -0-744 V
Ni deposited = (0.479 8 g - 0.477 5 g) = 2.3 mg = 39.19 pmol Ni which would
require (39.19 x IO"6 mol Ni)(2 e7Ni)(96 485 C/mol) = 7.562 C. Percentage of
current going to reduction of Ni2+ = 100 x g ; 082 c = 94%. The remainder went
into reduction of H' to H2.
16-13.
When excess Br2 appears in the solution, current flows at a low applied potential
difference (0.25 V) in thc detector circuit by virtue of the reactions
anode:
2Br" -» Br2 + 2e"
cathode:
Br2 + 2e" -> 2 Br*
16-14.
A mediator shuttles electrons between analyte and the electrode. After being
oxidized or reduced by analyte, the mediator is regenerated at the electrode.
16-15.
(a)
0.005 C/s x 0.1 s - 0.000 5 C
0.000 5 C
_
,.g
. .
96 485C/mol = 5 - 2 X l 0 " 9 m o , e
(b)
A 0.01 M solution of a two-electron reductant delivers 0.02 moles of
electrons/liter.
0.02 X molerte" = 2<>* ^
16-16.
(a)
L
= 0-0002 6 mL = 0.26 uL
, /'I
(5.32 x lQ-3 C/s)(964 s)
mol e = y =
96 485 C/mol
=
5 32
'
..,
*
10
mo1
220
Chapter 16
(b) One mol e" reacts with lÁ mol Br2, which reacts with lA mol cyclohexene
=> 2.66 x io -5 mol cyclohexene.
(c) 2.66 * IO"5 mol/5.00 x IO"3 L = 5.32 x IO' 3 M
16-17.
21" —• I2 + 2c" ^> one mole of I2 is created when two moles of electrons flow.
(812 s)(52.6 x 10-3 C/s)/(96 485 C/mol) = 0.442 7 mmol of c" = 0.221 3 mmol
of I2. Therefore, there must have been 0.221 3 mmol of H2S (FM 34.08) = 7.542
mgofH 2 S/50.00mL = 7.542 x 103 pgofH 2 S/50.00mL = 1 5 1 pg/mL.
16-18.
(a)
C6H5N=NC6H5 + 4H+ + 4e" ->
2C6H5NH2
Electron flow =
f.
electrons Y^^nmofyV
C\
4
25 9
96485
[ C6H5N=NC6H5A - "s~Jl
WîJ
• 'OOx 10-2C/s
1.00 x 1Q-2A
current density = t 0 Q x i 0 -4 m 2 = l-00xl0 2 A/m 2
=> overpotential = 0.85 V
(b) £(cathode) = 0.100 - 0.059 16 log [Ti0 2+] s [ H +]2
- 0 . 1 0 0 - 0.059 16 log ^ ' ^ ^ ^
(c) O2 + 4H + + 4e" ^= 2H 2 0
rt ~* x
1 n™ 0 059 16,
I
£(anode) = 1.229
¿
l°ß />02[H*]4
1 -,^
0059 16.
1
• L 2 2 9 - — T " l o * (0.20)[0.10]4 = , ]
-0.036 V
£ = 1.229 V
6 0 V
(d) £ =£(cathode) - £(anode) - /•/? - Overpotential
= -0.036 - 1.160 - (1.00 x 10- 2 A)(52.4O) - 0.85 = -2.57 V
-,
1A
16-19.
„
coulombs
b - ——--;—- =
I't
r
mol
mol
[0.203 639 0(±0.000 000 4) A1Í18 000.075 (±0.010) s]
" [4.097 900 (±0.000 003) g]/[ 107.868 2 (±0.000 2)g/mol]
_ [0.203 6390(±1.96 IQ-4%)][I8 000.075 (±5.56 10'5%)1
[4.097 900 (±7.32 10 5 %)]/[ 107.868 2 (±1.85 10"4%)]
- 9.648 667 x IO4 (±2.85 x IO"4 %) = 964 86.67 ±0.28 C/mol
221
Electroanalytical Techniques
I.
(a) H 2 S0 3
pATt = 1.86
.
HSO3
pAW.17
==
2SO-3
H 2 S03 is predominant below pH 1.86. HSO3 dominates between pH 1.86
and 7.17. SO^" is dominant above pH 7.17.
(b) Cathode:
H 2 0 + c- -» VM2{g) + OH"
Anode:
31" -> I3 +2e"
(c) I3 + HSO3 + H 2 0 -> 31- + SO4" + 3H*
O
O
I3 + 2S20 3 == 31- + 0 = S - S - S - S = 0
O"
O"
Thiosulfate
Tetrathionate
2
(d) In Step 3,13 was generated by a current of 10.0 mA (=10.0 x IO 3 C/s) for
4.00 min (= 240 s).
charge = It = (10.0 x K)-3 C/sX240 s) = 2.40 C
mol e- = I/F= (2.40 C)/(96 485 C/mol) = 24.8? pmol e~
The anode reaction generates Vi mol I3 for 1 mol e\ Therefore, 24.87 P-mol e*
will generate '/2(24.87) = 12.44 pmol I3.
In Step 5,0.500 mL of 0.050 7 M thiosulfate = 25.3s F-mol S2023". But 2 mol
S2023- consume 1 mol I3. Therefore, 25.3s umol S2023- consume lAÇ.S3$) 12.6s pmol I3.
We added excess SjO^* in Step 5 and consumed the excess in Step 6. In Step
6, we had to generate I3 at 10.0 mA for 131 s to react with excess S2023".
charge - It = (10.0 x 10"3 C/s)(131 s) = 1.31 C
mol e- = / / £ = (1.31 C)/(96 485 C/mol) = 13.5g pmol er
mol I3- - '/2( 13.5s) - 6.79 pmol I 3
Here is where we are so far:
Step 3: 12.44 pmol I¡ were generated.
Step 4: x pmol I3 were consumed by sulfite in wine.
Step 5: We added enough S2023- to consume 12.6s pmol I3.
Step 6: We had to generate 6.79 pmol I3 to consume excess S2023" from
Step 5. Therefore, I3 left after Step 4 = 12.6s - 6.79 = 5.89 pmol I3.
222
Chapter 16
We began with 12.44 pmol I3 and 5.89 pmol I3 were left after reaction with
sulfite in wine. Therefore, sulfite in wine consumed 12.44
5.89 = 6.55 pmol
I3. But 1 mol I3 reacts with 1 mol sulfite. Therefore, thc wine contained 6.55
pmol sulfite in the 2.00 mL injected for analysis.
Thc wine sample prepared in Slep 1 consisted of 9.00 mL wine diluted to
10.00 mL. Therefore, the original wine contained 10.00/9.00 of the amount
found in the analysis. That is, 2.000 mL of pure wine contains
(10.00/9.00)(6.55 pmol sulfite) = 7.28 pmol sulfite.
sulfite in wine =
7.2s pmol sulfite
2 00 mL
= 364
mM
This problem left out a description of the blank titration that should be done
in a real analysis. There are components in wine in addition to sulfite that
could react with I3. For the blank titration, 1 M formaldehyde is added to the
wine to bind all sulfite. The sulfite-formaldehyde adduct is not decomposed
in 2 M NaOH and does not react with [3. The blank titration consists of
taking this formaldehyde/wine solution through the entire procedure. We
subtract I3 consumed by the blank from I3 consumed by the wine without
formaldehyde.
16-21.
(a) Balance carbon: ß = c; balance halogen: C = x; balance nitrogen: £> = »
Balance oxygen: o + A = 2B => o + A=2c => A = 2c-o
Balance hydrogen: h + 2A = 3D + £ => h + 2(2c - o) = 3w + £
=> E = h +
4c-2o~3n
Charge b a l a n c e , £ = £ - C = A + 4 c - 2 o - 3 « - c = /i-c/2 + o - 3 n
(b) To consume Fe- requires F/4 0 2 , because each 0 2 consumes 4e".
(c) F = (9.43 x IO-3 Cy(9.648 5 x IO4 C/mol) = 9.774 x 10-8 m o l e mol 0 2 = £/4 = 2.223 x IO 8 mol
(d) The mass of 0 2 in (c) is (2.223 x 10-8 mol)(32.00 g/mol) = 7.11 4 x IO'7 g.
This much O2 was required to react with 13.5 pL of sample. The mass of 0 2
that would react with 1 L of sample is (7.11 4 x IO'7 g)/(13.5 x IO"6 L) =
0.0527 g/L - 52.7 mg/L.
(e) The balanced equation oxidation half-reaction is
C9H6N02ClBr2 + 16H20 - • 9C0 2 + 3X" + NH3 + 35H* + 32c'.
Electroanalytical Techniques
223
Thc observed number of electrons in thc reaction was 9.774 x IO"8 mol e% so
there must have been (9.774 x IO"8 mol e")/(32 mol e"/mol C 9 H 6 N0 2 ClBr 2 ) =
3.054 x IO"9 mol C9H6N02ClBr2 in 13.5 pL. The molarity of
C9H 6 N0 2 ClBr 2 is (3.054 x IO'9 mo)/(13.5 x IO 6 L) = 2.26 x IO' 4 M.
16-22.
The Clark electrode measures dissolved oxygen by reducing it to H2O at a gold tip
on a platinum electrode held at - 0.75 V with respect to Ag| AgCI. The opening of
the body of thc electrode is filled with a 10- to 40-pm-long plug of silicone rubber
that is permeable to 0 2 . Current is proportional to thc concentration of dissolved
O2 in the external medium. The electrode needs to be calibrated in solutions of
known 0 2 concentration.
16-23.
(a)
The glucose monitor has a test strip with two carbon indicator electrodes and
a silver-silver chloride reference electrode. Indicator electrode 1 is coated
with glucose oxidase and a mediator. When a drop of blood is placed on the
test strip, glucose from the blood is oxidized near indicator electrode 1 by
mediator to gluconolactone and the mediator is reduced. With a potential of
+0.2 V (with respect to the Ag| AgCI electrode) on thc indicator electrode,
reduced mediator is re-oxidized at the indicator electrode. The current
between indicator electrode 1 and the reference electrode is proportional to
the rate of oxidation of the mediator, which is proportional to the
concentration of glucose plus any interfering species in thc blood. Indicator
electrode 2 has mediator, but no glucose oxidase. Current measured between
indicator electrode 2 and the reference electrode is proportional to the
concentration of interfering species in thc blood. The difference between thc
two currents is proportional to the concentration of glucose in thc blood.
(b) In the absence of a mediator, the rate of oxidation of glucose depends on the
concentration of O2 in thc blood. If [0 2 ] is low, the current will be low and
the monitor will give an incorrect, low reading for the glucose concentration.
A mediator such as l.l'-dimethylferroccne can replace 0 2 in thc glucose
oxidation and be subsequently reduced at thc indicator electrode. The
concentration of mediator is constant and high enough, so variations in
electrode current are due mainly to variations in glucose concentration. Also,
by lowering the required electrode potential for oxidation of the mediator,
there is less possible interference by other species in the blood.
224
Chapter 16
(c) Glucose oxidase is replaced by glucose dehydrogenase, which docs not use
O2 as a reactant. The enzyme oxidizes glucose and reduces the PQQ cofactor
to PQOH2. PQQH2 is oxidized back to PQQ by a nearby Os3+ bound to the
polymer chain. A nearby Os2+ can exchange electrons with the Os3*. By
moving from Os to Os. electrons eventually reach thc carbon electrode. Thc
coulomctric sensor measures thc total number of electrons needed to oxidize
all of the glucose in the small blood sample.
(d) Amperometry measures current during the enzyme-catalyzed oxidation of
glucose. Current is proportional to the rate of the oxidation reaction. The
rates of most chemical reactions increase with increasing temperature.
Therefore, the current will increase with increasing temperature of the blood
sample. Coulometry measures the total number of electrons released in the
oxidation. Glucose releases 2 electrons per molecule, regardless of
temperature. The coulometric signal should have no temperature dependence.
(e) 1.00 g glucose/L = 5.55 mM glucose. A volume of 0.300 x IO'6 L contains
1.665 nmol glucose. Each mole of glucose releases 2e" and 2H* during
oxidation. Therefore, 2 x 1.665 nmol = 3.33 nmol e" are released. The
charge is Q = nF= (3.33 x IO-9 mol)(96 485 C/mol) = 321 pC.
16-24.
(ù is the rotation rate in radians per second. We need to convert rpm (revolutions
per minute) to radians per second.
(
. ^ revolutionsVl minY2ft radians^
,t
mM
l 2 - 0 0 x , 0 3 ~ m i n — A l Ô T A r e ^ i u f i o ^ J = 2 0 9 rad/s = 2 0 9 s - '
(because radian is a dimensionless unit)
2
6 = 1.61Z)1'W"
= 1.61(2.5 x 10- 9 m 2 /s) l/3 (l.I x 10-6 m 2/ s ) 1/6(209 rad/s)- m - 1.53 x i o 5 m
To calculate current density, we need to express the concentration of the species
reacting at the electrode in mol/m3 instead of mol/L. Since 1 L is the volume of a
10-cm cube, there are 1 000 L in 1 m3. Thc concentration of K^FeiCN^ is 50.0
mM - (0.O5OO x ) ( l 000 ¿ ) = 50.0 mol/m3.
Current density - 0.62«£Z)2/3v"l/6col/2Co
= 0.62(.)(96 485¿)(2.5x , 0 - ^ T ( L ' * ' ^ > " 5 > S
= 7.84 x IO2 - 5 - = 7.84 x 102 A¿
nr•s
m
225
Electroanalylieal Techniques
16-25.
(b)
(a)
/cu*
1
(Cu(Hg)
J
Cu* -> Cu (Hg)
o
/
C u 2 * -> Cu*
_L
-0.6
•0.3
-0.3
V
-0.6
V
(c) The potential for the reaction Cu(I) -> Cu(Hg) will change if Pt is used, since
the product obviously cannot be copper amalgam.
16-26.
(a)
Charging current arises from charging or discharging of the electric double
layer at the electrode-solution interface. Faradaic current arises from
oxidation or reduction reactions.
(b) Charging current decays more rapidly than Faradaic current. If we wait 1 s
after a potential step, thc charging current decays to near zero and the
Faradaic current is still significant. The ratio of the desired signal (Faradaic
current) to thc undesired background (charging current) is larger at 1 s than it
was at earlier times. If we wait too long, both signals become too small to
measure.
(c) In square wave voltammetry, an anodic pulse follows each cathodic pulse and
the signal is thc difference between the two. Thc anodic pulse oxidizes the
product of each cathodic pulse, thereby replenishing the electroactivc species
at the electrode surface for the next pulse. The concentration of analyte
available at the electrode surface is therefore greater in square wave
voltammetry.
16-27.
Electrons flowing in 3.4 min =
( 1 4 x 1 Q-6 C/s)(60s/minX3.4 min)
96 485 C/mol
R
¿.9 6 * I V
mo1 c
For the reaction Cd2* + 2e- -» Cd(in Hg),
moles of Cd 2 * =
2
moles of c" = 1.4s * 10"8mol
moles of Cd2* in 25 mL of 0.50 mM solution = 1.25 x 10"5 mol
1.48x io- 8
2
percentage of Cd * reduced = x 2 g x 1Q . 5 x 100 - 0.11 8 %
226
16-28.
Chapter 16
[X]i
ix
[S]f+[X]f-/s+X
x(mM)
^2.00^
/5Q.Q>
3.00
+ jr
l52.0OJ
16-29.
0.37 nA
0.80 pA
U2.0j
JC = 0.096 m M
In anodic stripping voltammetry, analyte is reduced and concentrated at the
working electrode at a controlled potential for a constant time. Thc potential is
then ramped in a positive direction to reoxidize thc analyte, during which time
current is measured. Thc height of thc oxidation wave is proportional to the
original concentration of analyte. Stripping is the most sensitive voltammctric
technique because analyte is concentrated from a dilute solution. The longer the
period of concentration, the more sensitive is the analysis.
16-30.
(a)
Concentration (deposition) stage: Cu2+ + 2e- -* Cu(.y)
(b)
Stripping stage: Cu(s) —> Cu2* + 2e-
(c) All solutions were made up to the same volume, so [X]¡ - [X]f•x. Prepare
a graph of/ vs. [S]f using data measured from the figure in the problem. The
intercept is at-313 ppb, so the original concentration of Cu2* is 313 ppb.
Added standard (ppb)
0
100
200
300
400
500
1.6
Current (//A)
0.599
0.774
0.943
1.12«
1.314
1.544
-
1.4
1.2
I
1.0
0,8
'f
3
Intercept =
-313 ppb
u^
-300
-200
-
" * y • 0.001 866 x + 0.5839
0,6;
"6.4
-
0.2
-100
100
200
300
[SJf(ppb)
400
500
Elcclroanalytical Techniques
D
E
A
B
C
Standard
Addition
Constant
Volume
Least-Squares
Spreadsheet
1
2
x
y
Relative
Added Fe(lll)
3
peak height
(DM)
4
0
1.00
5
50
1.56
6
1.98
100
7
B10
:C
12
=
UN
EST(B5:
B7,
A5:
A7.TRU E,TRU
8
E)
LINEST
output:
9
1.0233 b
m
0.0098
10
0.0522 sb
0.0008
11
sm
R2
0.0572 Sy
0.9932
12
-104.422
13 x-intercept = -b/m =
3 B14 = COUNT(A6:A7)
14 n =
1.513 B15 = AVERAGE(B5:B7)
15 Mean y =
2
5000 B16 = DEVSQ(A5:A7)
16 -r(Xi - mean x) =
17 Std deviation of
13.174
18 x-interceDt=
A
19 B18= (C12/ABS(B10))*SQRT((1/B14) + B15 2/(B10*2*B16))
y=0.0098x+1.0233
1.8
1 B
1 4
1,2
VI
Intercept
104 p M
09
06
04
\
120 -100
-80
BO
-40
?(\
2C
40
60
SO
00
Added Fe (pM)
Cells B13 and B18 of the spreadsheet tell us that [Fe(lll)] = 104+13 pM.
16-32.
Peak B:
RNHOH -> RNO + 2H+ + 2e"
PeakC:
RNO + 2H+ + 2e" - • RNHOH
There was no RNO present before the initial scan.
228
Chapter 16
16-33.
y=15.127x + 0-120
0.1
0.2
0.3
04
0.5
0.6
12
[Scan rate (V/s)] '
/p = (2.69 x
W^n^ACD^v^
slope = (2.69 x 10 8 )* 3/2 4CD I/2
slope2
°*
"= (2.69 x lOZfinWC2
(15.1 x | Q ^ A A / V 7 ¡ ) 2
" (2.69 x IO8)213(0.020 1 x 10-4 m2)2( 1.00-3 M) 2
= 7 8 X 10
'
"10m2/s
16-34.
Microelectrodes fit into small places, are useful in nonaqueous solution (because
of small ohmic losses), and allow rapid voltage scans (because of small
capacitance), which permits the study of short-lived species. The low capacitance
gives a low background charging current, which increases the sensitivity to
analyte by orders of magnitude.
16-35.
Thc Nafion membrane permits neutral and cationic species to pass through to the
electrode, but excludes anions. It reduces thc background signal from ascorbate
anion, which would otherwise swamp the signal from dopamine.
16-36.
ROH + S0 2 + B -*
BH* + ROS0 2
H 2 0 + I2 + ROS0 2 + 2B
-»
ROSO3 + 2BH*I-
One mole of H 2 0 plus one mole of I2 are required for the oxidation of the alky I
sulfite to an alkyl sulfate in the second reaction.
229
Electroanalytical Techniques
16-37. The bipotentiometric detector maintains a constant current (-10 pA) between two
detector electrodes, while measuring the voltage needed to sustain that current.
Before the equivalence point, the solution contains I", but little I2. To maintain a
current of 10 pA, the cathode potential must be negative enough to reduce some
component of thc solvent system (perhaps CH3OH + e" ^ CH3O" + 2H2(#)). At
the equivalence point, excess I2 suddenly appears and current can be carried at low
voltage by thereactionsbelow. The abrupt voltage drop marks the end point.
Cathode:
I¡ + 2c- -> 3F
Anode:
31" - •
I3 + 2e*
CHAPTER 17
FUNDAMENTALS OF SPECTROPHOTOMETRY
17-1.
(a)
double
(b) halve
(c) double
17-2.
(a) E = h\ = hclX « (6.626 2 x 10-34 j s )(2.9979 x io 8 m s-'VíóSO x 10-9 m )
= 3.06 x 10 l9 J/photon = I84kJ/mol
(b) For X - 400 nm, E = 299 kJ/mol.
17-3.
v = c/X = 2.9979 x io 8 m s-'/562 x 10-9 m = 5 33 x , 0 i 4
Hz
v - XIX = 1.78 K^m-' (1 m/IOOcm) = 1.78 104 cm -l
E = hv = (6.6262 x 10-34 j s)(5.33 x lO^s-') = 3.53 x 10 "^ J/photon
= 213 kJ/mol (after multiplication by Avogadro's number).
17-4.
Microwave energies correspond to molecular rotation energies. Infrared energies
correspond to vibrational energies. Visible light can promote electrons to excited
states (in colored compounds). Ultraviolet light also promotes electrons and can
even break chemical bonds.
17-5.
From the definition of index of refraction, we can write
^vacuum
=
n
' Cair
^•vacuum ' V • If • X^ - V
*-air ~ ^vacuum'"
v = c/Xvacmm = 5.088491 0 and 5.083 335 8 x I 0 , 4 H z
>-air = a-vacuunVn = 588.985 54 and 589.582 86 nm
Vair = l/>.air = 1.697834 5 and 1.696 1144 Kr+cm*1
17-6.
Transmittance (7) is the fraction of incident light that is transmitted by a
substance: T = P/P0, where P0 is incident irradiance and P is transmitted
irradiance. Absorbance is logarithmically related to transmittance: A = -log T.
When all light is transmitted, absorbance is zero. When no light is transmitted,
absorbance is infinite. Absorbance is proportional to concentration. Molar
absorptivity is the constant of proportionality between absorbance at a particular
wavelength and the product cb, where c is concentration and b is pathlcngth.
17-7.
An absorption spectrum is a graph of absorbance vs. wavelength.
17-8.
The color of transmitted light is the complement of the color that is absorbed. If
blue-green light is absorbed, red light is transmitted.
230
231
Fundamentals of Speclropholometry
Absorption
Predicted color
Curve
peak (nm)
(Table 17-1)
A
760
green
H
C
1)
i:
F
700
green
blue
violet
17-9.
600
530
500
410
Observed
color
green
blue-green
blue
violet
red
yellow
red or purple red
green-yellow
17-10. If absorbance is too high, too little light reaches the detector for accurate
measurement. If absorbance is too low, there is too little difference between
sample and reference for accurate measurement.
17-11.
e = Albc - 0.822/[(1.00 cm)(2.31 x IO"5 M)] = 3.56 x 104 M"1 cm-'
17-12.
Violet blue, according to Table 17-1.
17-13.
[Fe] in reference cell = Q o ^ 6 - 8 0
1(H) =
L36
*
]Qr M
Settin
* -
Sme
absorbanccs of sample and reference equal to each other gives e s 6 s c s » zrbxct.
But e s - er, so (2.48 cm)cs = (1.00 cm)(1.36 x 10-4 M) => c s = 5.48 x 10-5 M.
This is a 1/4 dilution of runoff, so [Fe] in runoff = 2.19 x 10"4 M.
17-14.
(a)
Measured from graph:
a = 1 . 3 x IO 2 0 cm2 at 325 nm
a - 3 . 5 * 10"19cm2 at 300 nm
at 325 nm: T = e" (8 x l o 1 8 cm"3X1-3 * l°-20<™2X1 cm) = 0 9 ( )
A = -log T = 0.045
at300nm: T = e"<8>< '0,8cm-3X3-5 * !0-,9cm2)(1 cm)
=
0061
A = - l o g T = 1.22
w
(b) T = e " *
0.14 - e " ( 8 X 10 l8 cm- 3 )a(l cm)
=> a = 2.4576 x 10- ,9 cm 2
..
.. 10/ r _ „-(7.92 x I018cm-3X2.4576X 10-|9cm2KI cm)
If n is decreased by 1%, T = e v
= 0.142 8
0.1428-0.14 _
Increase in transmittancc is
Q j^
- ¿.v/o.
Note that the fractional increase in transmittancc is greater than thc
fractional decrease in ozone concentration.
232
Chapter 17
(c) ^winter = e ~ ( 2 9 ° D U ) < 2 ' 6 9 * 1016 molecules/cm VD.U #2.5 * IO-'9Cm2)(l cm)
- 0.142
^summer = e ~ (350)(2.69 x 10^)<2.5 - 10 ^ K D
s
0.()95
Fractional increase in transmitlance is (0.142 - 0.095) / (0.095) = 49%.
17-15.
Neocuproine reacts with Cu(I) and prevents it from forming a complex with
ferrozinc that would give a false positive result in the analysis of iron.
17-16.
(a)
c = Aizb = 0.427/[(6 130 M"1 enr ' )( 1.000 cm)] - 6.97 x 10-5
(b)
The sample had been diluted x io => 6.97 x 10"4 M.
(C)
(292.16 g/moí)(e5.00x 10-3 L) = 6.97 x 10-4 M - ,
17-17.
Yes
17 i«
17-18.
M
á
(a) E = -h =
-.
(b)
17-19.
(a)
C =
A
eb
=
(3
x =
, 02
M
mg
0.267-0.019
.
,
. ] 5 x l 0 - 6 M ) ( L 0 0 0 c n i ) - 7.87 x IO4 * ! cur«
.
0.175-0.019
(7.87xlo4M-'cm')(1.000cm)
=
L9g
* ]0~* M
Thc absorbance due to the colored product from nitrite added to sample C is
0.967-0.622 = 0.345. The concentration of colored product due to added
•. •„ .
, « . (7.50 x lQ-3 MKlO.Qx I0-6U2
nitrite in sample C is *
ÖÖ54L
= 1.389 x | 0 ^ M .
e = A/bc = 0.345/[(l.389x lO^XS.OO)] - 4.97 x lO^M' 1 cm-'
(b) 7.50 x i o 8 mol of nitrite (from 10.0 pL added to sample C) gives
A • 0.345. In sample B, x mole of nitrite in food extract gives
A - 0.622-0.153 = 0.469.
x mol
7,50 x io- 8 mol
17-20.
=
0.469
Ö345 => x
=
1 0 2 0 x l0 7 m o 1 N 0
"
2 - 4.69 pg
(a) Prior to the equivalence point, all added Fe(III) binds to the protein to form a
red complex whose absorbance is measured in thc figure. After the
equivalence point, there are no more binding sites available on thc protein.
The slight increase in absorbance arises from the color of the iron titrant.
(b) 163 x IO"6 Lof 1.43 x 10-3MFe(III) = 2.33 x 10-7mol Fe(lII)
(c) 1.17 x IO-7 mol transferrin in 2.00 x 10-3 L => 5.83 x IO"5 M transferrin
233
Fundamentals of Spectrophotometry
17-21.
Theoretical equivalence point
0.003 57 g transferrin
mol Ga
transferrin;^
1
000
g transferrin/mol transferring
mol
(>
molGa
0.006 64
ë
Observed end point ~ intersection of lines taken from first 6 points and last 4
12.2
points in the following graph - 12.2 pL, corresponding to y j j = 91.7% of
2 Ga/transferrin - 1.83 Ga/transferrin. In the absence of oxalate, there is no
evidence for specific binding of Ga to the protein, since the slope of the curve is
small and does not change near 1 or 2 Ga/transferrin.
17-22.
(a) In the graph below, least-squares lines were put through the first 6 points and
the last 3 points. Their intersection is the estimated end point of 21.4 pL.
The moles of TCNQ in the titration are (0.700 mL)(l.00 x IO'4 M TCNQ) 70.0 nmol TCNQ. This many moles of Au(0) arc present in 21.4 pL of
nanoparticle solution. The mass of nanoparticles in 21.4 pL is
(21.4 pL)(1.43 g/L) = 30.6 pg nanoparticles. Tofindthe moles of Au(0)
in 1 00g of nanoparticles, set up a proportion:
x mol Au(0)
70.0 x IO'9 mol Au(0) _
30.6 x 10"" g nanoparticles 1.00 g nanoparticles
x = 2.29 mmol Au(0)
0.900 i
0.800
0.700
y = 003566X
+ 0.02182
O
O
y = -0.00005X
+ 0.7855
0.600
0.500
End point
= 21.4mL
0.400
0.300 •
0.200 .
0.100
Ti trant volume (8 L)
0.000
10
20
30
40
(b) 1.00 g of nanoparticles is estimated to contain 0.25 g C|2H2sS (FM 201.40),
which is 1.24 mmol C12H25S.
234
Chapter 17
(c) From (a), the mass of Au(0) in 1.00 g is (2.29 mmol Au(0Xl96.97 g/mol) =
0.451 g. The mass of Au(l) is estimated as the difference 1.00-0.451 0.25 = 0.299 g - 1.52 mmol Aii(I). The calculated mole ratio Au(I):C|2H2SS
is 1.52/1.24 = 1.23. Ideally, this mole ratio should be 1.00.
17-23.
n->Ti*(T|):
E = hv = h{ -(6.626 lx 1 0 - 3 4 j . s ) 2 ^ 9 M 0 ^ s - '
= 5 0 ( ) x 1().19j
To convert to J/mol, multiply by Avogadro's number:
5.00 x io-' 9 J/molccule x 6.022 x io2 3 molecules/mol = 301 kJ/mol.
»-«•(Si):
2 9979 x IO8 s-'
E = (6.6261 x 1 » * J » ) % S ^ i a * m
= 5.6OX io-' 9 J = 337kJ/mol.
The difference between the T| and S| states is 337 - 301 = 3 6 kJ/mol.
17-24.
Fluorescence is emission of light with no change in the electronic spin state of the
molecule (for example, singlet -> singlet). In phosphorescence, the electronicspin does change during emission (for example, triplet -+ singlet).
Phosphorescence is less probable, so molecules spend more time in the excited
state prior to phosphorescence than to fluorescence. That is, phosphorescence
has a longer lifetime than fluorescence. Phosphorescence also comes at lower
energy (longer wavelength) than fluorescence, because the triplet excited state is
at lower energy than thc singlet excited stak-.
17-25.
Luminescence is light given off after a molecule absorbs light.
Chemiluminscence is light given off by a molecule created in an excited state in a
chemical reaction.
17-26.
In Rayleigh scattering, electrons in molecules oscillate at the frequency of
incoming radiation and emit that same frequency in all directions. The time scale
is ~10' s for visible light. In Raman scattering, molecules extract a quantum of
vibrational energy from incoming light and scatter light with less energy than thc
incoming light. Again, the time scale is ~10"15 s for visible light. Fluorescence
occurs in 10"8 to 10"4 s, which is IO7 to IO11 times longer than scattering.
17-27.
Phosphorescence is emitted at longer wavelength than fluorescence. Absorption
is at shortest wavelength.
235
Fundamentals of Spectrophotometry
17-28.
In an excitation spectrum, the exciting wavelength (XçX) is varied while the
detector wavelength (^em) is fixed. In an emission spectrum, X^x is held constant
and Xem is varied. The excitation spectrumresemblesan absorption spectrum
because emission intensity is proportional to absorption of the exciting radiation.
17-29.
The spreadsheet computes relative fluorescence given by
relative intensity = j ^
- MT**«<1 - « T ^ f a ^ H T * * 6 .
A
|
B
C
Anthracene
fluorescence
response
1
2
1
1
e
9000 M" cm
3
ex ~
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
e
1
em —
b,=
02 =
b3 =
50 M-'cm"
0.3 cm
0.4 cm
0.5 cm
Relative
fluorescence
lntensity/c
l/k'Po
c(M)
8288
1.E-08 8.288E-05
8288
2.E-08 0.0001658
8286
4.E-08 0.0003314
8284
6.E-08I 0.000497
8282
8.E-08 0.0006626
8281
1.E-07 0.0008281
8272
2.E-07 0.0016544
8255
4.E-07 0.0033019
8238
6.E-07 0.0049426
8221
8.E-07 0.0065764
8203
1.E-06 0.0082034
8118
0.0162369
2.E-06
7951
4.E-06 0.0318052
7788
6.E-06 0.0467266
7628
8.E-06 0.0610222
7471
1.E-05 0.0747124
6738
2.E-05 0.1347552
5489
4.E-05 0.2195664
4482
6.E-05 0.2689283
8.E-05 0.2934519
3668
3009
1.E-04 0.300872
1154
2.E-04 0.2307768
196
4.E-04 0,0783318
38
6.E-04 0.0230136
8
8.E-04 0.0065982
2
1.E-03 0.0018832
O.OOOO 0.0002 0.0004 0.0006 0.0008 0.0010
Concentration (M)
Fluorescence increases with concentration
and then decreases because of selfabsorption. Most of the absorption occurs
at the excitation wavelength, and a little
comes at the emission wavelength.
Column C of the spreadsheet gives the
relative fluorescence intensity divided by
concentration. If intensity were
proportional to concentration, then column
C would be constant. We see that it is
constant at low concentration, and falls by
- 5 % at -5 pM. Thc calibration curve in
the text goes up to 0.6 pM, which is in the
linear range.
236
Chapter 17
17-30.
The equation for standard addition is
V
&+X I y
- / X + rJIcS],
Function to plot
on »'-axis
a
Function to plot
on.v-axis
where V0 is the volume of unknown in the cuvet (2.00 mL), Vs is the volume of
standard added (0 to 40 pL), Fis the total volume of unknown plus added
standard, [S]¡ is the initial concentration of standard (1.40 pg Se/mL), [X]¡ is thc
initial concentration of unknown in the 2.00-mL solution, / x is thc fluorescence
intensity from the unknown, and /s+x is the fluorescence intensity from the
unknown plus standard addition. We make a graph of/s+x VIV0 versus [S]¡
Fs/F0 in the following spreadsheet.
1
2
3
4
5
6
1
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
A
B
c
n
I
Standard Addition Variable Volume Least-Squares Spreadsheet
E
F
Vo (ml_) =
Vs (mL) =
X
y
2.00 volume
[SJi (ug/ml_) =
standard
Total volume x-axis function l(S+X) = y-axls function
1.40 added
V = Vo + Vs Si*VsA/o
signal
l(s+x)*V/Vo
0.0000
2.000
0.00000
41.4
41.4
0.0100
2.010
0.00700
49.2
49.4
0.0200
2.020
0.01400
56.4
57.0
0.0300
2.030
0.02100
63.8
64.8
0.0400
2.040
0.02800
70.3
71.7
B16:D18 = LINEST(F7:F11,D7: D11,TRUE,TR JE)
LINEST output:
m
1084.6
s„,
14.9
R'
0.9994
^^^^~"
41.670 b
0.255 Sb
0.330
h
x-intercept
- -b/m =
-Ó.03842I
n=
Mean y =
I(x, - mean x)2 =
5 B22 = COUNT(B7:B11)
56.8546 B23 = AVERAGE(F7:F11)
0.00049 B24 = DEVSQ(D7:D11)
2b
2b Std deviation of
27 K-lntercept =
0.000732
28 B27 =(C18/ABS(B 16))*SQRT((1/B22) + B23A2i (B16A2*B24))
237
Fundamentals of Spectrophotometry
!J.:::
/û
GO
y = 1084.6X +41.67
50
I
30
20
10
-0.040
-0.030
-0.020
-0.010
0.000
0.010
0.020
0.030
x = S,*V,/V0
The x-intercept is found from the equation of the straight line by setting .y = 0:
0 = 1084.6x + 41.67 => x = -0.038 42. The concentration of Se in the unknown
is 0.038 42 pg/mL. All Se from 0.108 g of Brazil nuts was dissolved in 10.0 mL
of solvent, which contained (10.0 mL)(0.038 42 pg/mL) = 0.384 2 pg Se. The
wt% Se in the nuts is 100 x (0.384 2 x IO'6 g/0.108 g) = 3.56 x 10-4 ^o/^
The standard deviation of the x-intercept is
H
Standard deviation _ £v_
of x-intercept
~\m\^\Jn
v2
2
m2>Z(x[-x)
where Sy is the standard deviation of.y, m is the slope, n is the number of data
points (= 5), y is the mean value ofy for the 5 points, x¡ are individual values of x,
and x is the mean value of* for thc 5 points. Cell B27 of the spreadsheet gives a
standard deviation of 0.000 732 for the x-intercept.
Therelativeuncertainty in the intercept is 0.000 732/0.038 42 = 1.91%. This is
the relative uncertainty in wr% Se if other sources of error are insignificant.
Uncertainty in wt% = (0.0191)(3.56 x IO"4 wt%) = 6.8 x IO"6 wt%. Answer =
3.56 (±0.068) x 10-4 wt%.
The confidence interval is ± / x (standard deviation) where t is Student's / (Table
4-2) for n - 2 = 3 degrees of freedom. The 95% confidence interval is
±(3.182)(0.068 x 10"4 wt%) = ±0.22 x 10"4 wt%. The value r = 3.182 was taken
from Tabic 4-2 for 3 degrees of freedom. Answer = 3.56 (±0.22) x IO"4 wt%.
CHAPTER 18
APPLICATIONS OF SPECTROPHOTOMETRY
18-1.
Putting b = 0.100 cm into the determinants gives
0.233 387
1640 0.233
0.200 642
1640 387
[X] =
399
399 0.200
1640 387
= 8.03 x 10-5 M [y] =
642
399
- 2.62 x 10-4 M
642
The spreadsheet solution looks like this, with answers in column F:
A
I
B
|
C
|
D
El
Analysis of a mixture by spreadsheet matrix operations
1
2
F
G
I
3
4
5
6
Wavelenqth
272
327
7
Coefficient Matrix
1640
399
Absorbance
of unknown
387
642
K
Concentrations
in mixture
0.233
0.2
8.034E-05
2.616E-04
A
c
<-[Xl
<-[Y]
18-2.
A
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
18-3.
B
c
D
E
F
G
I
H
Analysis of a Mixture When You Have More Data Points than Components of tho Mixture
Measured
Calculated
Absorbance
AbsorbWaveAbsorbance of Standard
of Mixture
Molar Absorptivity
ance
lenqth
Mn04
Cr207
Am
Mn04
Cr207
Acalc
[Acalc-Am]A2
266
0.042
0.410
0.766
420.0
4100.0
0.7650
1.017E-06
288
0.082
0.283
0.571
820.0
2830.0
0.5723
1.763E-06
320
0.168
0.158
0.422
1660.0
1580.0
0.4217
1.132E-07
350
0.125
0.318
0.672
1250.0
3180.0
0.6706
2.050E-06
360
0.181
0.056
560.0
0.366
1810.0
0.3690
9.106E-06
sum =
Standards
(Mn](M)=
1.00E-04
[Cr](M)=
1.00E-04 I
Pathlenqth
(cm) =
1.000
1.405E-05
Concentrations in the mixture
(to be found by Solver)
[Mn041 = 8.356E-05
[Cr207] >
1.780E-04
E6 = B6/($A$19*$A$14)
F6 = C6/($A$19*$A$16)
G6 = E6*$A$19'$D$14+F6*$AS19*SD$15
H6-ÍÜO U6|iA2
If the spectra of two compounds with a constant total concentration cross at any
wavelength, all mixtures with the same total concentration will go through that
same point, called an isosbestic point. The appearance of isosbestic points in a
238
239
Applications of Spectrophotometry
chemical reaction is good evidence that wc are observing one main species being
converted to one other major species.
18-4.
As V 0 2 + is added (traces 1-9), the peak at 439 decreases and a new one near 485
nm develops, with an isosbestic point at 457 nm. When V0 2+ /xylcnol orange >
1, the peak near 485 nm decreases and a new one at 566 nm grows in, with an
isosbestic point at 528 nm. This sequence is logically interpreted by the
sequence
M
+
L
-»
ML
439 nm
ML
485 nm
+
485 nm
M
->
M2L
566 nm
where M is vanadyl ion and L is xylenol orange. The structure of xylcnol orange
in Table 11-3 shows that it has metal-binding groups on both ends of the
molecule, and could form an Vb'- complex.
18-5.
Convert Tto A (= -log T) and then convert A to e (= Albc = /f/[(0.005)(0.01)])
c(M-,cnr1)
2022
1993 cm-'
Absorbance
2022
1993 cm-1
A
B
0.5086
0.01144
0.09854
0.699 0
A
B
10170
228.8
1971
13 980
For thc mixture, ¿2022 = -log (0.340) = 0.468 5 and A1993 = -log(0.383) =
0.4168. Equation 18-6 gives [A] = 9.11 x 10~3 M and [B] = 4.68 x IO"3 M.
18-6.
1
2
3
4
5
6
7
8
9
10
11
12
13
A
I
B
C
D
E
F
Solvina Simultaneous Linear Equations with Excel Matrix 0 perations
I
Coefficient Matrix
TB
STB
11100
4800
455
485
7350
11200
13900
36400
545
K
I
Wavelenqth
MTB
18900
11800
4450
Absorbance
of unknown
0.412
0.350
0.632
A
H
G
Concentrations
in mixture
1.2194E-05
9.2953E-06
1.3243E-05
C
<-ÍTBl
<-ÍSTBl
<-[MTB]
1. Hiqhliqht block of blank cells reauired for solution (G5:G7)
0 Tvn* thfi formula "= MMULTfMINVERSEÍB5:D7i.E5:E7r
3 Press CONTROL+SHIFT+ENTER on a PC or COMMAND+RETURN on a Mac
240
Chapter 18
18-7.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
18-8.
A
B l e
D I E
F | G|
H
Solving 4 Simultaneous Linear Equations with Excel Matrix Operations
Wavelenqth
Coefficient Matrix
EthylAbsorbance
Cone, in
(urn) p-xylene m-xylene o-xylene benzene of unknown
mixture
12.5
1.5020
0.0514
0 0.0408
0.1013
0.0627
13.0
0.0261
1.1516
u 0.0820
0.09943
0.0795
13.4
0.0342
0.0355
2.532 0.2933
0.2194
0.0759
14.3
0.0340
0.0684
0 0.3470
0.03396
0.0761
K
A
C
1.
2.
3.
4.
I
p-xvlene
m-xylene
o-xylene
Elhylbz
Hiqhllqht block of blank cells required 1"or solution (H5:H8)
Type the formula "= MMULT(MINVERSE(B5:E8).F5:F8i"
Press CONTROL+SHIFT+ENTER on a PC or COMMAND+RETURN on a Mac
The answer appears In cells H5:H8
The quantity of Hin is small compared to aniline and sulfanilic acid. Calling
aniline B and sulfanilic acid HA, we can write
Initial mmol:
Final mmol:
K m
KaKb
B
+
2.00
2.00 -x
K
HA
DIP
1.500
.500-*
(lQ-3.232)(/fw/1 Q.4.60I)
(2.00 -JC)(1. 500
= 23.39
Kw
<w
-x)
-• 23.39 => x •-•• 1.372 mmol
pH = pKßHf + log 7 ¿ ^ T = 4.601 + log
2.00-1.372
- 4.26
1.372
For Hin we can write:
absorbance = 0.110 = (2.26 x 1()4)(5.00) [Hin] + (1.53 x IQ4) (5.00)[ln"].
Substituting [Hin] = 1.23 x IO" 6 -[In"] gives [In"] = 7.94 x lO-'and
[Hin] = 4.36 x IO'7. The Hcnderson-Hasselbalch equation for Hin is therefore
1KL
,~
„
,
pH = pKWn + log [Hin] ^ 4 - 2 6 - P^HIn + log
18-9.
7.94 x iQ-7
4 36
-
10.7
P^Hin = 4.00.
(a) ¿620 = ea"¿[Hln] + eg¿6[In2-]
¿434 - e ^ ^ H I n - l + e ^ ô f l n 2 - ]
The solution of these two equations is given by Equation 18-6 in the text:
[Hin-] =J5(A(,2osl$4b-A434$2(>b)
241
Applications of Spcctrophotomelry
[In2-]
^(A434e"62%b-Ab2oàA%b)
where D = Keffieh-«!&*)
Dividing the expression for [In 2 ] by the expression for [Hin*] gives
[In 2 -] _ ¿434e l 6 1 20 "
t HIn "l A620Z$4
¿62Q£143n4
- A4i4¿£¿
Dividing numerator and denominator on the right side by ¿434 gives
[in2-]
t" 1 "'] " RA^U
(b)
^ H 43 n 4'-^2 n 0
¿8S-*A¿¡$
- *220"
¿È> - *AS4n34
Mass balance for indicator: [Hin-] + [In2*] = F]„
Dividing both sides by [Hin"] gives
[Hlnl [In2-]
F hl
[Hin-] + [Hin] ~" [Hin-] => ' + * I n
==
F tn
[Hin-] =*
[Hln J
Fin
" Äm+1
Acid dissociation constant of indicator:
„
rin2-1fH+l
Ä,n
" [Hin-]
Substituting F|n/(Äin + 1) for [Hin"] gives
*
(c)
ln
[In 2 -][H+](r?, n +0
"
Fin
^
l
J
/CinFin
"" [H ](Ä, n +l)
+
Equation A in the problem defines R\n as [In2-]/[HIn-]. So,
ATln = r i ^ H " ] n H ] + 1 = /iln[H+] => [H+] = KlnIR]n
(d)
From the acid dissociation reaction of carbonic acid, we can write
*' -
[HCOjKH*]
[C0 2 (o?)] =>
LHCU
3J "
g|[CQ2(qg)]
[H+]
From the acid dissociation reaction of bicarbonate, we can write
[CQ2j][H+]
i^ H C O j ]
*2 ~
[HCO5]
*
[C
°3"]
;=
[H+]
Substituting in thc expression for [HCO3] gives
_ JgiJC2[C02(ay)]
[CO3J [H+]2
(e)
Charge balance:
[Na+] + [H+] = [OH-] + [Hin-] + 2[In 2 '] + [HCO3] + 2[C023-]
242
Chapter 18
F N a + [H+] =
*w
Fin
[H+] Ä|„ + 1
(f)
+
^InFm
K\[CQ2(aq)]
[H+](/fIn + 1 ) + '
[H+]
K\K2[C02(aq)]
[\V]2
From part (c) we know that [H+] = KhJR\n. We calculate Ä|n from part (a):
In
*Ag4'34" - £620 _
(2.84K8.00 I 0 3 ) - ( 0 )
" e6n2o-ÄAC4f4 " ( 1 7 ° IO 4 )-(2.84X1.90 IO3)
::
l 95
- «
[H+] = K\nIRln = (2.0 x 10-7)/l,958 = 1.02 x 10"7M
Substituting this value of [H+] into the mass balance in part (e) produces an
equation in which thc only unknown is [C02(aq)]m.
FNa + [H+] =
*w
F ln
K\nFin
^i[C02(flg)]
K{K2[CQ2(aq)]
+
+
+
+
[H ] Rm + 1
[H ](/?ln + 1)
[H+]
[H+]2
92.0 x 10- 6 +1.0 2 * IO 7 =
(6.7 x IO'15) (50.0 x 1Q-6)
(2.0 x 10-7)(50.0 x IQ-6)
7 +
+ 2
(l.02xl0- )
1958+1
(1.0 2 x 10- 7 )(1.95g+l)
(3.0 x \0-7)[CO2(gq)]
(3.0 x !Q-7)(3.3 x 1Q-I l)[CQ2(a<?)]
(1.02 * IO'7)
(1.02 *10- 7 ) 2
9.21 x 10-5 - 6.56 x 10-8 + i 6 9 * ]0 -5 + 6.62 x ]0"5 +
+ 2.94 [C02(aq)] + 0.001 9 [C02(a<?)]
=> [C02(a<?)] = 3.0 4 x 10-6 M
(g) The ions in solution are Na+, Hin", In2-, HCO3 , CO^, H + , and OH". We
know that [Na+] = 92.0 pM and [H+] = 0.10 pM. If the total cation charge
is 92.1 pM, thc total anion charge must be 92.1 pM, and the ionic strength
must be -92 pM ^ IO"4 M. (The ionic strength is not exactly 92.1 pM
because some anions have a charge of-2, which will increase the ionic
strength from 92.1 pM.) An ionic strength of IO"4 M is low enough that the
activity coefficients are close to 1.00.
We can calculate the exact ionic strength from the following expressions
derived above:
IS
[OH") = Jjpj - 0.07 ^M
P*1 - ¿TT = •*»•** I'"2"] - [ H W D - "•' * "
[HCO5] =
K\[C02(aq))
[H+]
= 2.94 [C02{aq)] = 8.9 pM
243
Applications of Spectrophotometry
[CO2"] = ^
l / :
y
) ]
= 0.001 9 [C02(ag)3 = 0.003 pM
1 -r-* 2
Ionic strength •
/ c\z¡ =
i
5 {rjía+ll^fH+HMOH-]!^^
2
= 125 pM
18-10.
The Scatchard plot is a graph of [PX]/[X] versus [PX]. Data in the following
spreadsheet are plotted in thcfigurein the textbook. The slope is -4.0 x IO9 M'1,
giving a binding constant K = 4.0 x IO9 M"1. The fraction of saturation in
column E is S = [PX]/P0, where Po - 10.0 nM. S ranges from 0.29 to 0.84.
A
|
B
|
C
D
E
F
P
=
10
nM
Data
for
Scatchard
plot
0
1
Fraction of saturation
2
= [PxyPo
PXMX]
3 [PX] (nM) [X] (nM)
0.29
24.0
0.120
2.87
4
0.38
19.8
0.192
3.80
5
0.47
15.7
0.296
4.66
6
0.55
0.450
12.3
5.54
7
0.63
629
0.731
8.61
8
0.68
5.54
6.77!
1.22
9
0.75
5.02
7.52
1.50
10
0.84
2.34
8.45
3.61
11
18-11.
(a)
F
G
E
B
C
D
A
1 Estradiol - Albunin Scatchard Plot
2 abscissa ordinate
Xo/X
Highlight cells B15:C17
P(+M)
3
1.26
Type
|
|
6.3
4
1.62
"=LINEST(B4:B12A4A12,TRUE.TRUE)"
5
10.0
2.16
Press CTRL+SHIFT+ENTER (on PC)
G
20.0
2.51
Press COMMAND* RETURN (on Mac)
7
30.0
8
9
40.0
50.0
10
11
12
13
eo.0
3-34
3.33
4.19
70.0
80.0
4.13
4.36
|
|
Student's t (95% confidence,
|
7 degrees of freedom) =
I 2.364624 =TINV(0.05.7)
95% confidence interval =
0.008248 = r s m = D11*B16
14
15
16
LINEST output
m 0.042885 1.243476 b
8k> 0.003488 0.166224 Sb
17
R2 0.955742
0.259413
&
244
Chapter 18
y=0.0429x+ 1.2435
[P] biM
The slope is 0.042 88 with a standard deviation of 0.003 49 in cells B15 and
B16. However, the units on the abscissa are pM, so the slope is really
(0.042 88 ± 0.003 49)/10"6 M"1 = (4.288 ± 0.349) x IO4 M'1. To find the 95%
confidence interval, we need Student's t for 9 - 2 = 7 degrees of freedom,
which is / = 2.365 in cell D i l . The 95% confidence interval is
(0.349)(2.365) = 0.825. The final result is K = (4.3 ± 0.8) x IO4 M 1 .
(b) Estradiol is X. The quotient [X]o/[X] is 1.26 at the first point and 4.36 at the
last point. The fraction of free estradiol is [X]/[X]0 = 1/1.26 = 0.79 at the
first point and 1/4.36 = 0.23 at the last point. Thc fraction of bound estradiol
is 1 - 0.79 = 0.21 at the first point and 1 - 0.23 = 0.77 at the last point.
18-12.
(a) We will make the substitutions [complex] = Ale and [\2] -
[\2]ioi-
[complex] in the equilibrium expression:
[complex]
Alt.
=
=
[h][mesitylene] "= ([^Jtot - [complex]) [mesitylene]
^[l2]tot - ^[complex] = — —
J
e[mesitylene]
Making the substitution [complex] = Ale once more on the left-hand side
gives
*[l2]tot-— = 8 r m e s i t y | c n c ]
Multiplying both sides by e and dividing by [\2]t0\ gives the desired result:
KA
A
eK
[l2]tot " [bltot [mesitylene]
245
Applications of Spectrophotometry
(b) Thc graph of /f/([mesitylcne][l2]tot) versus Al[l2]t0i is an excellent straight
line with a slope of-0.464 and an intercept of 4.984 x IO3. Since slope =
-K, thc equilibrium constant is 0.464. The molar absorptivity is e =
intercept//^ => e = 1.074 x 104 M"1 cur 1 .
18-13.
After running Solver, the average value of K in cell E10 is 0.464 and e =
1.073 x IO4 M"1 cm"1. The Scatchard plot in the previous problem gave
K - 0.464 ande = 1.074 x 10 4 M 1 cm- 1 .
E
A
B
| C
D
1 Equilibrium constant for reaction of l 2 with mesitylene
2
[Complex] = A/e
A
Keq
3 [Mesitylene] (M) [l2]tot(M)
7.82E-05 0.369
3.437E-05 0.46443
1.6900
4
2.56E-04 0.822
7.657E-05 0 46349
0.9218
5
0.787
7.331 E-05 0.46439
3.22E-04
0.6338
6
6.549E-05 0.46473
3.57E-04
0.703
7
0.4829
0.3900
3.79E-04 0.624
5.813E-05 0.46480
8
0.3271
3.93E-04 0.556
5.179E-05 0.46353
9
Average = 0.46423
10
Standard Dev = 0.00058
11 Guess for e:
Stdev/Average = 0.00125
1.073E+04
12
13
14 D4 = C4/$A$12
15 E4 = D4/(A4*(B4-D4)) = [com plex]/([Mesitylene][Free 2 »
16 E10 = AVERAGE(E4: E9)
17 E11 = STDEV(E4:E9)
18 E12 = E11/E10
19 Use Solver to va rye (cell A15Î) until c ell E12 is minimized
18-14.
(a) Maximum absorbance occurs at XscN- = 0.500
=> stoichiomctry = 1 : 1 (n — 1)
0.4
'—i—'—r
0.3
-e 0.2
8
0.1
o
0
0.2
i
i
0.4
0.6
L
i
0.8
Mole fraction SCN"
1
246
Chapter 18
(b) The curved maximum indicates that the equilibrium constant is not very
large.
(c) The different acid concentrations give both solutions the same ionic strength
(=16.0mM).
18-15.
Thc Job plot peak is at a xylenol orange mole fraction of 0.40, suggesting thc
stoichiometry (xylenol orange^Zra which has a mole fraction of
xylenol orange
xylenol orange + Zr(IV) " 2 + 3 - 0.4
The plot could have been improved by obtaining data points at mole fractions of
0.35 and 0.45 to verify the location of the maximum.
0.500
8
0.400
n»
•E 0.300
n
«0
S 0.200
-^
jo
«
0.100
0.000
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
08
0.9
Mole fraction xylenol orange
18-16.
(a)
Here are the results:
[A]total
[B] total
le-5
2e-5
2.5e-5
3e-5
3.33e-5
4e-5
5e-5
6e-5
7e-5
8e-5
9e-5
9c-5
8e-5
7.5e-5
7e-5
6.67e-5
6c-5
5e-5
4e-5
3e-5
2e-5
le-5
K --• 10<>
8.01e-8
1.26e-7
1.39C-7
1.45e-7
1.46C-7
1.42e-7
1.23e-7
9.49e-8
6.24e-8
3.18e-8
8.97e-9
[AB2]
K--••IO?
7.27e-7
1.14e-6
1.25e-6
1.30e-6
1.31e-6
1.28e-6
1.12e-6
8.66e-7
5.78e-7
3.00e-7
8.68e-8
K = 10« Mole fraction A
4.02e-6
0.1
6.26e-6
0.2
6.83e-6
0.25
7.12e-6
0.3
7.17e-6
0.333
6.99e-6
0.4
6.20e-6
0.5
4.97e-6
0.6
3.51e-6
0.7
2.00e-6
0.8
6.70C-7
0.9
247
Applications of Spectrophotometry
(b) The maximum occurs at a mole fraction of A = 1/3, since thc stoichiometry
is 1:2. The greater the equilibrium constant, the greater the extent of
reaction and the steeper thc curve. When thc equilibrium constant is too
small, thc curve is so shallow that it does not at all resemble iwo intersecting
lines.
0.2
0.4
0.6
0.8
Mole fraction of A =[AJ/([A]+[B])
0.0
18-17.
The mole fraction of thymine varies from 0.10 to 0.90 in increments of 0.10 as
we go down the table. Job's plot reaches a broad peak at a mole fraction of 0.50,
which is consistent with 1:1 complex formation. Job's plot gives us no
information on the structure of the product except for its stoichiometry.
0.4
I
o
0.3
—'
I
:
g
c
o
u
a
I
•—T
'
-
2
.
0
'
V1.
/
0.1
1
AUN -.
— 0.2
Cl
o
'
i
0.2
.
i
1
0.4
0.6
.
1
.
0.8
1
Mole fraction of thymine derivative
248
18-18.
Chapter 18
Inßow injection analysis, unknown is injected into a continuously flowing
stream that could contain a reagent which reacts with analyte. Alternatively, one
or more reagents can be added to the flow downstream of sample injection. The
sample and reagents travel through a coil in which mixing and reaction occur.
Absorbance or fluorescence or some other property of the flowing stream is
measured in thc detector. In general, reactants and products do not reach
equilibrium in flow injection analysis. The precision of the result depends on
executing the same process in a very repeatable manner.
In sequential injection, unknown and one or more reagents are sequentially
injected under computer control into a holding coil. There is not a continuous
flow. At an appropriate time, flow is reversed and the mixture is directed through
a detector. As in flow injection analysis, equilibrium is generally not reached and
precision depends on repeatability of the sequence of steps.
The principal difference between flow injection and sequential injection is
that there is continuous flow in the former and there is a sequence of steps
without continuous flow in the latter. Sequential injection uses less reagent and
generates less waste than flow injection. Sequential injection is called "lab-on-avalve" because sample, reagents, and product all flow through a central multiport valve.
18-19.
Each molecule of analyte bound to antibody 1 also binds one molecule of
antibody 2 that is linked to one enzyme molecule. Each enzyme molecule
catalyzes many cycles of reaction in which a colored or fluorescent product is
created. Therefore, many product molecules are created for every analyte
molecule.
18-20.
In time-resolved emission measurements, the short-lived background
fluorescence decays to near zero prior to recording emission from the lanthanide
ion. By reducing background signal, the signal-to-noise ratio is increased. Also,
the wavelength of the lanthanide emission is longer than the wavelength of much
of the background emission.
249
Applications of Spectrophotometry
18-21.
5 um
latex
bead
Step 1
Step 3
Step 2
S
o*
antibody
Fluorescence
labeled TNT
d
TNT
In Step 1, antibodies for TNT are attached to latex beads. In Step 2, the antibody
is saturated with a fluorescent derivative of TNT. Excess fluorescent derivative
is removed. In Step 3, thc beads are incubated with TNT, which displaces some
fluorescent derivative from binding sites on the antibodies. The suspension of
beads is then injected into the flow cytometer. As each bead passes in front of
thc detector, it is excited by a laser and its fluorescence is measured. The graph
in the textbook shows median bead fluorescence versus TNT concentration in a
series of standards. The more TNT in the standard, the less fluorescence remains
associated with the beads.
18-22.
Thc graph of Ksv versus pH has a plateau at low pH near Ksv ~ 100 and a plateau
at high pH near Ksv ~ 1350. Thc quencher, 2,6-dimethylphenol, is a weak acid
whose pKa is expected to be near 10. A logical interpretation is that the basic
form, A", is a strong quencher with Ksv ~ 1350, and the acidic form, HA, is a
weak quencher with Ksv ~ 100. Wc estimate pKa as the midpoint in the curve at
ATSV ~ (1350 - IO0)/2 = 625. At this point, pH a 10.8, which is our estimate for
pK; The literature value is 10.63.
The smooth curve in the graph is a least-squares fit to the equation
Aw
LSV
—
A. -,,
' ÍHÍL
+ Ka)
+ K
™[[H+] + Ka)
Fraction in form HA Fraction in form A"
Quenching by HA
The least-squares fit gave
and pA'a = 10.78.
KHSA
Quenching by A'
- 69.0 M-1, K% = 1370M" 1 ,
250
18-23.
Chapter 18
(a) The following graph at the left shows that thc Stern-Volmer equation is not
obeyed. If it were obeyed, the graph would be linear.
7
V = 4.399x + 0.014
0.2
0.3
Q(rrM)
0.4
0.5
0.0 i
0.2
0.3
0.4
0.5
Q(mM)
(b) The graph at the right above shows that Equation 4 is obeyed. Ideally, the
intercept should be zero. The slope of thc graph is AW([S] - [CMC]).
Given that [Q] was expressed in mM, we will express [S] and [CMC] in
mM: 4.399 = Nav I ([20.8]- [8.1 ])-+ Nav = 55.9
(c) [M] = ([S]-[CMC])/AT av = ([20.8]-[8.1])/55.9 = 0.227 mM
Q = [QMM] = 0.200 mM / 0.227 mM = 0.881 molecules per micelle
(d) P« - ^ r e - Q -
For« = 0, P 0 = fr°Ml
P, - ^ e - 0 . ™
- 0.365
= 0.414
ft.^l-a. 61
CHAPTER 19
SPECTROPHOTOMETERS
19-1.
The light source provides ultraviolet, visible, or infrared radiation. The
monochromator selects a narrow band of wavelengths to pass on to the sample.
As the experiment progresses, thc monochromator scans through a desired range
of wavelengths. The beam chopper is a rotating mirror that alternately directs
light to the sample or reference. The sample cuvet holds thc sample of interest.
The reference cuvet is an identical cell containing pure solvent or a reagent
blank. Thc mirror after thc reference cuvet and the semi transparent mirror after
the sample cuvet pass both beams of light through to the detector, which could be
a photomultiplier tube that generates an electric current proportional to the
photon irradiance. The amplifier increases thc detector signal for display.
19-2.
An excited state of the lasing material is pumped to a high population by light, an
electric discharge, or other means. Photons emitted when the excited state decays
to a less populated lower state stimulate emission from other excited molecules.
The stimulated emission has thc same energy and phase as the incident photon.
In the laser cavity, most light is retained by reflective end mirrors. Some light is
allowed to escape from one end. Properties of laser light: monochromatic,
bright, collimatcd, polarized, coherent
19-3.
Deuterium. Silicon carbide globar.
19-4.
Resolution increases in proportion to thc number of grooves that are illuminated
and to the diffraction order. The number of grooves can be increased with a more
finely ruled grating (closer grooves) and with a longer grating. The diffraction
order is optimized by appropriate choice of the blaze angle of the grating.
Dispersion is proportional to the diffraction order and inversely proportional to
the spacing between lines in the grating. The closer thc lines, the greater the
dispersion.
The optimum wavelength selected by a grating is thc one for which thc
diffraction condition nX = a\sm Q + sin <))) is satisfied by specular reflection
when the angle of incidence is equal to the angle of reflection (a • ß).
19-5.
A filter removes higher order diffraction (different wavelengths) at the same
angle as thc desired diffraction.
251
252
Chapter 19
19-6.
Advantage - increased ability to resolve closely spaced spectral peaks.
Disadvantage - more noise because less light reaches detector.
19-7.
(a) A photomultiplicr tube has a photosensitive cathode that emits an electron
when struck by a photon. Electrons from the cathode are accelerated by a
positive electric potential toward the first dynode. When an accelerated
electron strikes the dynode, more electrons are emitted from the dynode.
This multiplication process continues through several successive dynodes
until ~10 6 electrons arc finally collected at the anode for each photon
striking thc photocathode. The signal is the current measured at the anode.
(b) Each photodiode in a linear array has /Mype silicon on a substrate of n-type
silicon. Reverse bias draws electrons and holes away from the junction,
which is a depletion region with few electrons and holes. The junction acts
as a capacitor, with charge on either side of the depletion region. At the
beginning of a measurement cycle, each diode is fully charged. Free
electrons and holes created when radiation is absorbed in the semiconductor
migrate to regions of opposite charge, partially discharging the capacitor.
Charge left in each capacitor is measured at the end of a collection cycle by
measuring the current needed to recharge each capacitor.
(c) A charge coupled device is made from/»-doped Si on an «-doped substrate.
Thc structure is capped with an insulating layer of S1O2, on top of which is a
two-dimensional pattern of conducting Si electrodes. When light is absorbed
in thc ¿»-doped region, an electron is introduced into the conduction band and
a hole is left in the valence band. The electron is attracted to the region
beneath the positive electrode, where it is stored. The hole migrates to thc
«-doped substrate, where it combines with an electron. Each electrode can
store ~10 5 electrons. After the desired observation time, electrons stored in
each pixel of the top row of the array are moved into a serial register at the
top and then moved, one pixel at a time, to the top right position, where the
charge is read out. Then the next row is moved up and read out, and the
sequence is repeated until the entire array has been read.
19-8.
DTGS has a permanent electric polarization. That is, one face of the crystal has a
positive charge and the opposite face has a negative charge. When the
temperature of the crystal changes by absorption of infrared light, the polarization
253
Spectrophotometers
(the voltage difference between the two faces) changes. The change in voltage is
the detector signal.
19-9.
(a) nX = ¿/(sin 0 + sin (|>)
1 • 600 x IO-9 m = úfYsin 40' + sin (-30°)) => d = 4.20 x 10"6 m
Lines/cm = 1/(4.20 x îO^cm) = 2.38 x 103 lines/cm
(b) ÎL = 1/(1 000 c m 1 ) = 10"3cm => d = 7.00 x 10 3 cm => 143 lines/cm
19-10.
19-11.
103 grooves/cm means d = 1 0 - 5 m = 10 pm
n
1
A , M radians
Dispersion = rjLLLLL^ - ( 1 0 ( i m ) c o s 1 0 ° - 0.102 ^ m
radians
180°
0. 0 2 — T — x
= 5.8°/pm
r
pm
n radians
X
512 245
(a) Resolution = 7J[ = 0 0 3 = 1.7 x 104
X
512 23
(b) AX = -¡-j = - j ¿ 4 - = 0.05 nm
(c) Resolution - nN = (4)(8.00cm x | 850 cm-1) = 5.9 x |{)4
(d) 250 lincs/mm = 4 pm/line = d
Aà
n
1
radians
,.,..
TT = -;
7 = 71
ï
« = 0 . 2 5 0 — — - = 4.3°/pm
AX dcosty
(4pm)cos3°
pm
For AA. = 0.03 nm, ¿4 = (14.3°/pm)(3 x 10-5 pm) = 4.3 x 10"4 degrees.
For 30th order diffraction, the dispersion will be 30 times greater, or 0.013°.
19-12.
(a) True transmittancc = IO' 1 - 500 = 0.0316. With 0.50% stray light, the
apparent transmittance is
P+S
0.0316 + 0.005 0
Apparent transmittance = o n + c ~
1 + 0 005 0
_
.....
0.030 4
The apparent absorbance is -log 0.036 4 = 1.439.
(b) Apparent absorbance = 1.999
Apparent transmittance = IO"1999 = 0.010 023 05
P+S
0.010 + s
Apparent transmittancc = pTTT =
1 + c ~ 0.010 023 05
=> S= 2.328 x IO"5 or 0.002 328%
(c) For true absorbance = 2,
P+S
Apparent transmittance = pn + $
=
0.010 + 0.000 000 5
nMnMAAtt
—1 + 0 000 000 5 = 0.010 (WO 49
254
Chapter 19
Apparent absorbance is -log T= -log(0.010 000 49) = 1.999 978
Absorbance error = 2-1.999 978 • 0.000 022
For true absorbance = 3,
P+S
0.001 +0.000 000 5
Apparent transmittance = p +s = l + o 000 000 5 = 0 ' ü 0 1
00
°495
Apparent absorbance is -log 7*= -log(0.001 000 495) - 2.999 785
Absorbance error = 3 - 2.999 785 = 0.000 215
19-13.
¿ = |r(l906-698cm-l)
=
0
-1242mm
(Air between the plates has refractive index of 1.)
19-14.
M = aT* - [5.6698 x 10-8 W(m2K4)]T4
M(Wm2)
7KK)
77
1.99
298
447
»-is. (» n - M É f — L . ^
at T = 1 000 K:
X(nm)
2.00
10.00
A4 (W/m93)
8.79 x io
1.164x109
(b) MkAX = (8.79 x io 9 w/m3)(0.02 x 10-* m) = 1.8 x io 2 W/m2 at 2.00 pm
(c) MxAX - (1.164 x IO9 W/m3)(0.02 x 10^m) = 2.3 x io 1 W/ m 2 at 10.00 pm
(d) atT=
100 K:
A/x(W/m3)
Mpm)
2.00
10.00
A*2.00 pm
Mo.OOpm
A/2.00 pm
77
A/|0.00P m
=
=
6.69 x io-' 9
2.111 x io 3
8.79 x IQ9 W/m3
1.164 x |0 9 W/m3 ~ 7 ' 5 5
6.69 x l Q - 1 9 W / m 3
0 111V i M w / 1 =
2.111 x IO'* W / m J
at l 00
3 1 7 x
°
K
IO" 22 at 100 K
At 100 K, there is virtually no emission at 2.00 pm compared to 10.00 pm,
whereas at 1 000 K, there is a great deal of emission at both wavelengths.
255
Spectrophotometers
í9 16
- ' ^-^ïôlj-t)
=
19-17.
0.210 m
f
(3.00 x 10 8 m/s)lnlOU6.06x 10-6 s 18.52 xlO' 6 s
-2.51x10-6
»isin8| = M2 sin O2, where «i = 1.50 and n2 = 1.33
(a) IfOi = 30\ 9 2 = 34*
(b) If 0i = 0°, 02 = 0° (no refraction)
19-18.
Light inside thc fiber strikes thc wall at an angle greater than the critical angle for
total reflection. Therefore, all light isreflectedback into the core and continues
to be reflected from wall-to-wall as it moves along the fiber. If the bending angle
is not too great, the angle of incidence will still exceed the critical angle and light
will not leave the core.
19-19.
When traveling from medium 1 into medium 2, the critical angle for total internal
reflection is sin Ocr¡tical = "2^1. where n{ is the refractive index of medium i.
For the solvent/silica interface, «i = 1.50 and n2 = 1.46, so sin 6critical = 1 -46/1.5
- 0.973 3. «critical= sin"'(0.973 3) = 1.339 radians from the Excel function
ASIN(0.9733). Degrees = 180 x • • • - 180 x ^ « 76.7°.
For the silica/air interface, nx = 1.45 and n2 • 1.00, so sin «critical = 1.00/1.46 0.684 9. Ocritical • sin"'(0.684 9) = 0.754 5 radians. Degrees = 180 x -'•j— =
43.2°.
The angle in the photograph is -55', which exceeds the critical angle at the
silica/air interface but does not exceed the critical angle at the solvent/silica
interface. Total internalreflectionin the photo must be from the silica/air
interface.
19-20.
Light is transmitted through the diamond crystal waveguide by total internal
reflection at thc upper and lower surfaces. The upper surface is in contact with a
fluid channel containing sorbent beads that retain caffeine from a soft drink
flowing through thc channel. Caffeine in the beads absorbs some of the
evanescent wave from the totally internally reflected radiation, decreasing the
radiant power transmitted through thc waveguide. The integrated area of the
absorption spectrum of transmitted power is proportional to the concentration of
caffeine in the soft drink.
256
19-21.
Chapter 19
Sensitivity increases as the number of reflections inside the waveguide increases,
because there is some attenuation at each reflection. For a constant angle of
incidence, the number of reflections increases as the thickness of the waveguide
decreases.
Three reflections
Six reflections in the same
length when waveguide
thickness is cut in half
r\SKSKJKS*z?*i;
19-22.
(a) The value of 0¡, called the critical angle (0C), is such that (ni/n2)sin 0C = 1.
For «| = 1.52 and »2 = 1.50, 0C = 80.7°. That is, 0 must be > 80.7° for
total internal reflection.
y ^ ^
(b)
19-23.
- lO-WB/myiO . io-(20.0mX0.0100dB/myiO
= 0 955
(a) WcoreSinOj = »cladding sin 0 r
For total reflection, sin 0r > 1 => sin 0¡ >
Wcladdm
fi
"core
For «cladding • 1.400 and « c o r e = 1.600, sin 0¡ >^QÔ
(b)
19-24.
^
0
¡ - 61.04°
For »cladding = 1.400 and Mcore - 1.800,0¡ > 51.06°
Angle of incidence = angle of reflection = 45*. Angle of refraction = 0.
«prism sin 45° = rta¡rsin0. If total reflection occurs, there is no refracted light.
•PI.-
u
-e • û
,
»prism SJtl 4 5 °
This happens if sin 0 > 1, or M n^
> 1. Using na„ = I gives
«prism > V2. As long as n p r ¡ s m > *Ji, no light will be transmitted through the
prism and all light will be reflected.
19-25.
(a) Thc Teflon tube acts as an optical fiber beeause the internal solution has a
higher refractive index (1.33) than the walls (1.29). The tube is a 4.5-m-long
sample cell that can be conveniently coiled to fit in a reasonable volume and
guide the incident radiation all the way through thc tube. The long
pathlength allows us to obtain a measurable absorbance for a very low
257
Spectrophotometers
concentration of analyte.
(b) «core s ' n Oi
=
"cladding sin 0 r
For total reflection, sin 0 r > I => sin 0¡ >
»cladding
«core
1.29
For Cladding = 1.29 and/i core = 1.33, sin 0¡ I f ^ => 0¡ I 76°
(c) A = ebc = (4.5 x 10 4 M-' cm-')(450 cm)(1.0 x 10"9M) = 0.020
19-26.
(a) Thc following diagram shows thc path ofa light wave through the
waveguide. The length of one bounce, t, satisfies thc equation (0.60 pm)/¿
= tan 20°, giving t = 1.648 pm. Thc hypotenuse of the triangle, h, satisfies
thc equation (0.60 pm)//i - sin 20*, giving h = 1.754 pm. The number of
intervals of length I in 3.0 cm is (3.0 cm)/( 1.648 pm) - 1.820 x 104.
Therefore, the pathlcngth covered by the light is h x (1.820 x IO4) = 3.19
cm.
0.60 pm
power out _
power in
1 0 _ i ( d B / m y| 0
_ ,Q-(3.19cm)(0.050dB/cm)/l0 = 0.964
(b) Wavelength = XJn, where X0 is the wavelength in vacuum and n is the
refractive index. Wavelength = (514nm)/1.5 • 343 nm.
The frequency is unchanged from that in vacuum:
v = cfX = (2.9979 x 10» m/s)/(514 x IO'9 m) - 5.83 x IO14 Hz
19-27.
/. ( u n i )
(a)
0.2
0.4
0.6
0.8
1
1.5505
1.4701
1.4580
1.453 3
1.4504
2
3
4
5
6
1.4381
1.4192
1.3890
1.340 5
1.258 0
(b) dnldX is greater for blue light (~ 400 nm) than for red light (~ 600 nm)
Chapter 19
1.6 i
«
1
r——|
.
,
,
'
'
'
3
'
1
r
l.o
1.4
"S
tl
Red
Blue
1.3
12 '
0
'
'
1
'
2
'
4
i
»
5
•
6
Wavelength (pm)
19-28.
19-29.
(a)
A = ±2 cm
(b)
Resolution refers to the ability to distinguish closely spaced peaks.
(c)
Resolution ¡a 1/A = 0.5 cm"1
(d)
S - l/(2Av) = 1/(2 x 2000 cm-«) = 2.5 pm
The background transform gives the incident irradiance P0. The sample
transform gives the transmitted irradiance P. Transmittance is P/P0, not P P0.
19-30.
White noise is independent of frequency. One source is random motion of
charge carriers in electronic circuits, l^noise decreases with increasing
frequency. Drift and flicker of the lamp ofa spectrophotometer are sources of
l//noise. Line noise results from disturbances at discrete frequencies. Thc 60
Hz wall frequency is the most common electromagnetic disturbance seen in
electronic instruments.
19-31.
In beam chopping, the beam is alternately directed through the sample and
reference cells. Slow drift of source intensity should be cancelled because the
intensity seen by the sample and reference are almost the same. More rapid
flicker of the lamp might not be cancelled.
19-32.
The difference voltage is ideally very close to zero if the sample and reference
are the same because thc same lamp intensity goes through each compartment.
If the sample absorbs some radiation, the difference voltage should respond to
sample absorption with very little noise from source flicker.
259
Spectrophotometers
19-33.
To increase the ratio from 8 to 20 (a factor of 20/8 = 2.5) requires 2.52 = 6.25
~ 7 scans.
19-34.
(a)
(100± 1) + (100± 1) = 200 ±-\/2, since«? = -\e] + e\ = ^\2+\2
- ^2
(b) (100± 1) + (100± 1) + (100± 1)+ (100± 1) = 400±2, since
e = ^ 2 + [2+ i2+i2 = 2. The signal-to-noise ratio is 400:2 = 200:1.
(c) The initial measurement has signal/noise = 100/1.
Averaging « measurements gives
«• 100
average signal = —~— = 100
average noise = „ = 1^/w
average signal _ 100
r1UU
average noise
\/^f, "
V">
which is "\[ñ times greater than the original value of signal/noise.
19-35.
The theoretical signal-to-noisc (S/N) ratio should increase in proportion to the
square root of thc number of cycles that are averaged.
Number of cycles - n
1
100
300
1 000
y¡ñ
1
10.00
17.32
31.62
Predicted relative S'N ratio
=1
10.00
17.32
31.62
If the observed S/N = 60.0 for the average of 1 000 cycles, then the predicted
S/N for thc other experiments are shown in the following table:
Number of cycles
[ 000
Predicted S/N ratio
Observed S/N ratio
60.0 (observed)
60.0
32 9
300
60.0 (jfÜ) "
-
100
1
60.0 @-f¡§ = 19.0
60.0 ( T T « ) = 1-90
35 9
-
20.9
1.95
CHAPTER 20
ATOMIC SPECTROSCOPY
20-1.
Temperature is more critical in emission spectroscopy, because the small
population of the excited state varies substantially as the temperature is changed.
The population of thc ground state does not vary much.
20-2.
Furnaces give increased sensitivity and require smaller sample volumes, but give
poorer reproducibility with manual sample introduction. Automated sample
introduction gives good precision.
20-3.
Drying (~20-100*C) removes water from the sample. Ashing (~T0O-500*C) is
intended to remove as much matrix as possible without evaporating analyte.
Atomization (-500-2 000*C) vaporizes analyte (and most of the rest of the
sample) for thc atomic absorption measurement. For some samples, ashing
temperatures might be much higher than 500°C to remove more of the matrix, but
it must be demonstrated that the ashing does not remove analyte.
20-4.
The plasma operates at higher temperature than a flame and the environment is
Ar, not combustion gases. The plasma decreases chemical interference (such as
oxide formation) and allows emission instead of absorption to be used. Lamps
are not required and simultaneous multi-element analysis is possible. Selfabsorption is reduced in the plasma because the temperature is more uniform.
Disadvantages of the plasma are increased cost of equipment and operation.
20-5.
Doppler broadening occurs because an atom moving toward the radiation source
sees a higher frequency than one moving away from the source. Increasing
temperature gives increased speeds (more broadening) and increased mass gives
decreased speeds (less broadening).
20-6.
(a) A beam chopper alternately blocks or exposes thc lamp to the flame and
detector. When the lamp is blocked, signal is due to background. When the
lamp is exposed, signal is due to analyte plus background. The difference is
the desired analytical signal.
(b) The flame or furnace is alternately exposed to a D2 lamp and the hollowcathode lamp. Absorbance from the D2 lamp is due to background.
Absorbance from thc hollow-cathode lamp is due to analyte plus
background. The difference is the desired signal.
260
261
Atomic Spectroscopy
(c) When a magnetic field parallel to the viewing direction is applied to the
furnace, the analytical signal is split into two components that are separated
from thc analytical wavelength, and one component at the analytical
wavelength. The component at the analytical wavelength is not observed
because of its polarization. The other two components have the wrong
wavelength to be observed. Analyte is essentially "invisible" to the detector
when thc magnetic field is applied, and only background is seen. Corrected
signal is that observed without a field minus that observed with thc field.
20-7.
Spectral interference refers to the overlap of analyte signal with signals due to
other elements or molecules in the sample or with signals due to the flame or
furnace. Chemical interference occurs when a component of the sample
decreases thc extent of atomization of analyte through some chemical reaction.
Isobaric interference is thc overlap of different species with nearly thc same
mass-to-chargc ratio in a mass spectrum. Ionization interference refers to a loss
of analyte atoms through ionization.
20-8.
La 3+ acts as releasing agent by binding tightly to P0 4 " and freeing Pb 2+ .
20-9.
(a) A collision cell guides ions to the entrance of the mass separator and reduces
the spread of ion kinetic energies by a factor of 10.
(b) A dynamic reaction cell contains a reactive gas such as NH3, CH4, NiO, CO,
or O2 and its electric field is configured to select lower and upper masses of
ions to pass through the cell. Plasma species which interfere with some
elements can be reduced by as many as 9 orders of magnitude by reactions
such as electron transfer (40Arl6O+ + NH3 — NH} + Ar + O) and proton
transfer C°ArH+ + NH3 - • NH4 + Ar). Thc reactive gas can also be used
to shift thc analyte signal from a position at which interference occurs (for
example, ^Ar^O 4 interferes with S6Fef) to one where there is no interference
(S6Fe+ + N 2 0 —
S6
Fe ,6 0 + + Nj).
(c) When 87 Sr + is converted to 87 Sr 19 F + , which has a mass of 106, it no longer
overlaps with 87 Rb + in thc mass spectrum.
20-10.
The extent to which an element is ablated, transported to the plasma, and
atomized depends on the matrix in which it is found. Different elements in a
given matrix might not behave in thc same manner. Thc most reliable calibration
262
Chapter 20
is for the analyte of interest to be measured in thc same matrix as the unknown—
if that is possible.
20-11.
In the excitation spectrum, we are looking at emission over a band of
wavelengths 1.6 nm wide, while exciting the sample with different narrow bands
(0.03 nm) of laser light. The sample absorbs light only when thc laser frequency
coincides with thc atomic frequency. Therefore, emission is observed only when
the narrow laser line is in resonance with the atomic levels. In thc emission
spectrum, the sample is excited by a fixed laser frequency and then emits
radiation. The monochromator bandwidth is not narrow enough to discriminate
between emission at different wavelengths, so a broad envelope is observed.
20-12.
ForPb:
Similarly, we multiply each of the other concentrations by 11.5 g snow/cm2 to
findTl: 0.005 ±0.001; Cd: 0.04 ±0.01; Zn: 2.0 ±0.3;
Al: 7 ( ± 2 ) x lOlng/cm 2 .
M ,20-13.
.
hç_ (6.626 x 10-34 j.s)(2,998 x io» m/s)
l
X = — =
= 5.893 x l0-7m = 589.3nm
3 3 7 | ;\ 0 .i9j
20-14.
We derive the value for 6 000 K as follows:
,F
.,
te
(6.626 1 x lQ-34 j . S){2,9979 x 10« m/s)
_
6E
l
~ hV = X =
500* 10-9 m
= 3.97X 10-lvj
— = &-e-AE/kT = s- e -(3.97x 10-19j)/(|.38i x |0-23J/KX6 000K) = ^ - ( 8 . 3 x IO"3)
O
SO
gO
gçy
3
Ií'g*/go = 3, then N*/N0 = 3(8.3 x 10" ) = 0.025.
20-15.
Doppler linewidth: AX = X (7 x
\0^)yJflM
For X = 589 nm, A/= 23 (sodium) at T= 2 000 K,
AX - (589nm)(7x 10-7)V(2000)/23 = 0.0038 nm
For X = 254 nm, A/= 201 (mercury) at T= 2 000 K,
AX = (254nm)(7x 10"7)>/(2000)/201 = 0.000 5 6 nm
263
Atomic Spectroscopy
he
20-16.
(a) AE = Av = J -
(6.626 1 x 10-34 j -s)(2.997 9 x 10« m/s)
422.7* 10* m
• 4.699 x 10-19 J/molecule = 283.0 kJ/mol
~v tH _ S^ e _A£/*7 , = 3e-(4.699x 10-'9jy(].38l
J*o
x
10*23 J/KX2 500K) = 3 ^ 7 x 10-6
£0
(c) At 2 515 K, iV*/Ao = 3.98 x 10"6 =^ 8.4% increase from 2 500 to 2 515 K
(d) At 6000 K, N*IN0 = 1.03 * IO"2
20-17.
Cu
Br
Element:
Na
8.04
3.78
2.10
Excited state energy (eV):
154
328
Wavelength (nm):
591
2/3
3
Degeneracy ratio (g*/g0):
3
1.4 x 10-7
1.8 x IO-16
iVV/Vo at 2 600 K in flame:
2.6x10-4
1.2 x IO 7
2.0 x IO"3
N*/N0 at 6000 K in plasma:
5.2 x lf>2
Calculations: wavelength = hclAE
N*IN0 * (g*fg0) <r*BtkT
Br is notreadilyobserved in atomic absorption, because its lowest excited state
requires far-ultraviolet radiation for excitation. Nitrogen and oxygen in the air
absorb far-ultraviolet energy and would have to be excluded from the optical
path. The excited state lies at such high energy that it is not sufficiently
populated to provide adequate intensity for optical emission.
20-18.
The dissociation energy of YC is greater than that of BaC, so the equilibrium
BaC + Y ^ Ba + YC is driven to the right, increasing the concentration of free
Ba atoms in the gas phase.
20-19.
Area of pit = n(20 x 10"1 cm)2 = 1.26 x IO"5 cm2
Power = 2.4 x IO'3 J/10 x IO*9 s = 2.4 x 10s W
Power density - 2.4 x 10s W/1.26 x 10s cm2 = 1.9 x 1010 W/cm2 = 20 GW/cm2
Ablated mass = volume x density = depth x area x density =
(1 x IO"4 cm)(1.26 x 10"5 cm2)(4 g/cm3) = 5 ng
20-20.
Analyte and standard are lost in equal proportions, so their ratioremainsconstant.
Chapter 20
A
B
C
1 Standard Addition Constant Volume Least-Squares
2
x
y
3 Volume added (mL) Absorbance
4
0.00
0.151
5
1,00
0.185
6
3.00
0.247
7
0.300
5.00
8
8.00
0.388
9
10.00
Ü.445
10
15.00
0.572
11
20.00
0.723
12 B14:C16 = =LINEST(B4:B11 ,A4:A11 JRUE.TRUE)
13
LINEST output:
14
m
0.0282
0.1579 b
15
Sn,
0.0003
0.0031 Sb
IT'
16
0.9993
0.0057
D
«y
17
18
19
20
21
22
23
x-intercept = -b/m =
-5.5991
n=
8 =COUNT(A4:A11)
Mean y =
0.376 = AVERAGE(B4:B11)
S(x¡ - mean xf =
343.5 = DEVSQ(A4:A8)
Std deviation of
x-lntercept =
0.1630
B22=(C16/ABS(B14))*SQRT((1/B18) + B19A2/(B14A2*B20))
The ¿-intercept is -5.60 ±0.16 mL. Standard [Ca] = 20.0 pg Ca/mL. The
intercept corresponds to Ca = (5.60 ±0.16 mL)(20.0 pg Ca/mL) = 112.0 ± 3.2
pg. This is the mass of Ca in 5.00 mL of unknown. The total volume of
unknown was 100.0 mL, so mass of Ca in total unknown = (100.0 mL/5.00
mL)( 112.0 ± 3.2 pg Ca) = 2240 ± 64 pg Ca.
^ „ .
, 100x(2240 ± 64 ug Ca)
=
wt% Ca in cereal =
0 ' 5 21 6 g cereal
0429 ±
°012
wt%
-
0.)!
0.6
8
06
0.4
Manan
5.60 m
0.3
1
00
-10
-5
0
5
Added Ca (mL)
10
15
20
265
Atomic Spectroscopy
20-22.
(a) [S] in unknown mixture = (8.24 pg/mL) ( ^ g ) = 0.824 pg/mL
Standard mixture has equal concentrations of X and S:
Ax JA£\
0^930
F
[*]
[X] - \iS]) => [*]
=/
pflOOgh
A[-s-]J
F =0.930
Unknown mixture:
Ax m JAs\ . 1.690
[X] - * ^[SU - [X] - 0 9 3 ° (l0.824mg/mLl) =* W " 1 4 9 7 ^ m L
But X was diluted by a factor of 10.00/50.0, so thc original concentration in
the unknown was (1.497 pg/mL) [TQ^OöJ
= 7 49
-
P-g/ml-
(b) Standard mixture has equal concentrations of X and S:
„(As)
0.930
p.OOO^
Ax
pq = 4[S]J => [342] - Flil.00]J =* F-0-W9
Unknown mixture:
(As\
1.690
Ax
ÏXÏ =
F
_._. f
l.OOO
^
y * IXT = 0-27l9l[0.824pg/mL]J *
rvl
et
-
[X] = 5 1 2 2
pg/mL
But X was diluted by a factor of 10.00/50.0, so thc original concentration in
the unknown was (5.122 pg/mL) ITQ^JQJ - 25.6 pg/mL.
20-23.
A
B
1 Least-Squares Spreadshee t
X
2
pg Kj'mL
3 Highlight cells B10:C12
Q
B4:B8.TRUE,TRUE)
5
5
D
C
C
F
1
y
signal
700
b For PC. press
1U
486
CTRL+SHIFT+ENTER
20
7
8 For Mac. press
COMMAND+RETURN LINEST outpul:
9
4.0259
m 23.7672
10
0.2256 3.6090
Sm
11
R" 0.9997 5.4338
I2
600
19
20
21
H
1
1
1
fiOfl
0
124
13
14
15
16
17
18
G
1
J
500
b
Sb
>. 400
300
-
•f
200
n=
Mean y—
S(x, - mean x) 2 =
5
UNT(B4:BÍ >>
B14 = CCidFICAC.fi
- AVER/
560 =DEVSÍ 3(B4:B6)
100
:
Measured y =
k = Number of replicate
measurements of y =
-
in
ic
on
417 Input
X
1 Input
Derived x = 17.3758 = (B18-C10J/B10
A
A
0.2539 = (C12rei0)*SQRT((1/B19K1/B14)+((B18.B15) 2V(B10 2*B16))
1
1
1
9i
-ln
266
Chapter 20
Cells B20 and B21 give us [unknown] = 17.4 ± 0.3 pg/mL for an emission
intensity of 417.
20-24.
(a) CsCI provides C s atoms which ioni/e to Cs + + e- in the plasma. Electrons in
the plasma inhibit ionization of Sn. Therefore, emission from atomic Sn is
not lost to emission from Sn+.
A
B
C
D
E
F
1 Tin in canned food - Anal. Bioanal. Chem. 2002, 374, 235
2 Calibration data for 189.927 nm
3
4 Cone (pg/L) Signal intensity
Output from LINEST
0
5
4.0
slope
[intercept
10
6
8.5
Parameter 0.781651 0.863321
7
20
StdDev
19.6
0.018508 1.556732
30
8
23.6
RA2
0.996648 3.213618
40
9
31.1
10
60
41.7
11
100
78.8
12
200
159.1
13
14 Select cells E6:F8
15 Enter the formula = LINEST(Bi>:B12,A5:A12.TRUE,TRUE)
16 CONTROL+S HIFT+ENTER on PC or COMMAND+RETURN on Mac
160
140
120
tl
100
80
en
60
40
23
0
0
50
100
150
200
(Sn](uoJL)
(c) For the 189.927 nm Sn emission line, spike recoveries are all near 100 pg/L,
which is near 100%. None of thc elements in thc table appears to interfere
significantly at 189.927 nm. For the 235.485 nm emission line, interference
from an emission line of Fe is so serious that the Sn signal cannot be
measured. Several other elements interfere enough to reduce the accuracy of
thc Sn measurement. These elements include Cu, Mn, Zn, Cr, and, perhaps,
267
Atomic Spectroscopy
Mg. The 189.927 nm line is clearly the better of thc two wavelengths for
minimizing interference.
(d) Limit of detection = minimum detectable concentration = 3slm
where s is the standard deviation of thc replicate samples and m is the slope
of thc calibration curve. Putting in thc values s = 2.4 units and m = 0.782
units per (pg/L) gives
3(2.4 units)
35
limit of detection = - = 0 .782 units/(pg/L) "
9 2
^
L
10ä
. .
,
. •
10(2.4 units)
„
limit of quantitation = — = 0 .782 units/(pg/L) == 30J WL
It would be reasonable to quote a limit of detection as 9 pg/L and a limit of
quantitation as 31 pg/L.
(e) A 2-g food sample ends up in a volume of 50 mL. The limit of quantitation
is 30.7 pg Sn/L for the solution. A 50-mL volume with Sn at the limit of
quantitation contains (0.050 L)(30.7 pg Sn/L) = 1.54 pg Sn. The quantity of
Sn per unit mass of food is
(1.54 uESnXlmg/1 000 pg)
(2.0 g foodX 1 kg/1 000 g) "
20-25.
0J7
mgSn
kg food
= ( ? 7 7 p p m
u
/
pm
'
P
Standard addition graph: plot signal versus Ti or S concentration.
Signal
S (ppm)
0.0174
0.0
0.0221
37.0
0.0268
74.0
148.0
0.0362
Ti (ppm) Signal
0.86
0.00
1.10
3.00
1.34
6.00
1.82
12.00
2.00
1.80
0.04
y = 0.08000X + 0.86000
y = 1.270E-04X + 1.740E-02
0.035
/
1.60
0.03
1.40
0.025
1.20
re
E
/
1.00
n 0.80
g>
0.02
0.015
0.60
0.01
0.40
/
0.005
0.20
0.00
-15
-10
-5
0
p i (ppm)]
5
10
15
•*
-150-100 -50
0
50
(S(ppm)]
100 150 200
268
Chapter 20
Ti standard addition graph: negative intercept = 0.860/0.0800 = 10.75 mg/L
S standard addition graph: negative intercept = 0.017 4/0.000 127 - 137.0 mg/L
Ti atomic mass = 47.867
S atomic mass - 32.065
[Ti] - (10.75 mg/L)/(47.867 g/mol) - 2.246 x IO"4 M
[S] = (137.0 mg/L)/(32.065 g/mol) = 4.273 x 10-3 M
[Transferrin] = [S]/39 = 1.096 x IO"4 M
Ti/transferrin = (2.246 x 10^» M)/(1.096 x IO"4 M) - 2.05
CHAPTER 21
MASS SPECTROMETRY
21-1.
Gaseous molecules are ionized by collisions with 70-cV electrons in the ion
source. The ions are accelerated out of the source by a voltage, V. All ions have
nearly the same kinetic energy (2 mv2> where m is mass and v is velocity), so the
heavier ions have lower velocity. Ions then enter a magnetic field (B) and are
deflected so they travel through the arc ofa circle whose radius is
(^¡2V(m/z)/e)/B, where z is the number of charges on the ion and e is the
elementary charge. By varying the magnetic field, ions of different m/z are
deflected through thc slit leading to the detector. At the detector, ion impacts
liberate electrons from a cathode. Thc electrons are amplified by a series of
dynodes (as in a photomultiplier tube). The mass spectrum is a graph of detector
signal versus m/z.
21-2.
For the electron impact spectrum, pentobarbital is bombarded by electrons with
an energy of 70 electron volts. The molecular ion (m/z = 226) produced by the
impact has enough energy to break into fragments and little M+* is observed.
Large peaks correspond to the most stable cation fragments. For chemical
ionization, pentobarbital reacts with CHj, which is a potent proton donor, but
docs not have excess kinetic energy. The dominant peak is usually MH+ (m/z =
227). In the case of pentobarbital, some fragmentation is observed even in the
chemical ionization spectrum.
21-3.
fll/12)x 12 g/mol (exactly)^
B l -U
1 dalton(Da) = 1/12 ofthe mass of «^C = ^ 6.022 14 * IO23 mol"' J
= 1.660 54 x 10"24g
[5.03 (±0.14) x 1010 Da][1.660 54 x IO' 24 g/Da]
= 8.35 (+0.23) x 10- 14 g = 83.5 (±2.3) fg
21-4.
The atomic mass in the periodic table is a weighted average of the masses of all
the isotopes ofthat element. We can estimate the relative abundance of the two
major isotopes of Ni from the heights of their mass spectral peaks. The heights
of the peaks that I measured from an earlier version of this illustration are 42.6
mm for 58 Ni and 17.1 mm for 60 Ni. Thc weighted average is
269
270
Chapter 21
atomic mass
= (58Ni mass)(% abundance of 58 Ni) + (60N¡ mass)(% abundance of 60 Ni)
- (57-935 3)( 4 2 f+6]7A)
+ (59.933 2 ) ( ^ 6 %
T
) = 58.51
The atomic mass in thc periodic table is 58.69. This main reason for
disagreement is that we neglected the existence of61 Ni (1.13% natural
abundance), 62 Ni (3.59%), and ^Ni (0.90%).
21-5.
Resolving power = —•
=
Q 19
= 1.5 x IO4.
We should be able to barely distinguish two peaks differing by 1 Da at a mass of
1.5 x io 4 Da. Therefore, we should be able to distinguish two peaks at 10 000
and 10 001 Da.
21-6.
The overlap at the base of the peaks is approximately 10% in the mass spectrum.
The resolving power is approximately mlAm » 31/0.010 ~ 3 100.
21-7.
Resolving power by 10% valley formula: mlAm = 906.49/0.000 45 = 2.0 x IO6
Resolving power by half-width formula: m/mm = 906.49/0.000 27 = 3.4 x io 6
The mass of an electron, 0.000 55 Da, is greater than thc mass difference between
the two compounds. The mass difference of the compounds is 82% of the mass
of one electron.
21-8.
C5H70+:
5x]2.000 00
+7x 1.007 825
-e- mass
C6H!+,:
-e- mass
+1 x 15.994 91
-1 x 0.Q0Q55
83.049 14
6x12.000 00
+ 11 x 1.007 825
-1 x 0.000 55
83.085 52
C6Hj+, is a closer match than C 5 H 7 0 + to thc observed mass of 83.086 5 Da.
271
Mass Spectrometry
21-9.
31
P+ =
3l
P - e- = 30.973 7 6 - 0 . 0 0 0 55 = 30.973 21 (observed: 30.973 5 )
To measure m/z, I enlarged thc figure and sketched a Gaussian curve over each
signal by eye. I then measured the position of the center of the peak with a
millimeter scale ruler.
15N160+ = l 5 N + 1 6 0 - e- = 15.000 11 + 15.994 91 - 0 . 0 0 0 55
= 30.994 47 (observed: 30.994 6 )
4
l N O H + - l 4 N + 1 6 0 + 'H - e- =
14.003 07 + 15.994 91 + 1.007 8 2 - 0 . 0 0 0 55 = 31.005 25
(observed: 31.0036)
21-10.
l6
79
8
Br abundance • a = 0.506 9
' Br abundance • b - 0.493 1
79
2
Abundance of C 2 H2 Br 2 = a - 0.256 9s
Abundance of C 2 H 2 7 9 B r 8 ' B r = 2ab - 0.499 9 0
Abundance of C 2 H 2 8 l B r 2 = b2 = 0.243 1 5
Relative abundances: M + : M+l : M+2 = 1 : 1.946 : 0.946 3
Figure 21-7 shows the stick diagram.
21-11.
10
B abundance = a = 0.199
Abundance of 1 0 B 2 H 6 = a2 = 0.039 6 0
Abundance of
10
l,
B abundance = b = 0.801
B n B H 6 - 2ab = 0.318 8
Abundance of , l B 2 H 6 - b2 - 0.641 6
Relative abundances: M + : M+l : M+2 = 1 : 8.05 : 16.20
R + DB = c - A/2 + w/2 + 1
21-12. (a)
R + DB = 11 -18/2 + 2/2+ 1 = 4
phénobarbital, C I I H I 8N2O3
(b)
The molecule has onering+ three double
bonds.
B r * ^ / ^ . P(CH3)2
R + DB = c - A/2 + nl2 + 1
A
1
HO A YySCH 2 CH3
R + DB
" j^j
C[2H|5BrNPOS
. 15+1 1 + 1 .
£
• 1 2 - ^ - +"2~+1 =6
The molecule has two rings + four double
bonds. Note that h includes H + Br, and n
includes N + P. S, like O, does not
contribute to the count.
272
Chapter 21
(c)
R + DB = c-hl2 + nl2+l
R + DB = 3 - 5/2 + 1 = 11/2 Huh?
II
A fragment in a mass specirum
21-13.
C6H5CI: M + *=I12
(a) ((J\—C]
<
We come out with a fraction instead of an
integer because the species is an ion in
which one C makes three bonds instead of
four.
^
The pair of peaks at m/z = 112 and 114 strongly suggest that the molecule
contains 1 CI.
rings + double bonds = c - A/2 + nl2 + 1 = 6 - 6 / 2 + 1
=4
Î
A includes 11 +CI
Expected intensity of M+l is 1.08(6) + 0.012(5) = 6.54%
carbon
hydrogen
Observed intensity of M+l = 69/999 = 6.9%
Expected intensity of M+2 = 0.005 8(6)(5) +32.0(1) = 32.2%
carbon
chlorine
Observed intensity of M+2 = 329/999 = 32.9%
The M+3 peak is the isotopic partner of the M+2 peak. M+3 contains 37CI
plus either 1 13C or 1 2 H. Therefore, the expected intensity of M+3 (relative
to M+2) is 1.08(6) + 0.012(5) = 6.54% of predicted intensity of M+2 =
(0.065 4X32.2) = 2.11% of M+*.
Observed intensity of M+3 is 21/999 = 2.1 %.
(b) C l — ( I
G
))—C\
C6H4C12: M+'=146
The peaks at m/z - 146, 148, and 150 look like the isotope pattern from 2 Cl
in Figure 21-7.
rings + double bonds = c-hl2 + nl2 + 1 = 6 - 6 / 2 + 1 = 4
Expected intensity of M+l is 1.08(6)+ 0.012(4) = 6.53%
carbon
hydrogen
Observed intensity of M+I = 56/999 = 5.6%
Expected intensity of M+2 = 0.005 8(6)(5) + 32.0(2) • 64.2%
carbon
chlorine
273
Mass Spectrometry
Observed intensity of M+2 = 624/999 = 62.5%
The M+3 peak is thc isotopic partner of thc M+2 peak. M+3 contains 1 35C1
+ 1 37C1 plus either I l3C or I 2 H. Therefore, the expected intensity of M+3
(relative to M+2) is 1.08(6) + 0.012(4) = 6.53% of predicted intensity of
M+2 = (0.065 3)(64.2) = 4.19% of M+*.
Observed intensity of M+3 is 33/999 = 3.3%.
Expected intensity of M+4 from C6H437C12 is 5.11(2)(1) = 10.22% of M + \
The small contribution from I2C4|3C2H435C137C1 is based on the predicted
intensity of M+2. It is 0.005 8(6)(5) = 0.174% of 64.2% = 0.11 %.
Total expected intensity of M+4 is 10.22% + 0.11% - 10.33% of M+"
Observed intensity = 99/999 = 9.9%.
Expected intensity of M+5 from l2C513CH437Cl2 and I2C6H32H37C12 is
based on the predicted intensity of M+4. M+5 should have 1.08(6) +
0.012(4) = 6.53% of M+4 = 6.53% of 10.33% = 0.67%.
Observed intensity = 5/999 - 0.5%.
(c) \ Q ) —
NH
2
C6H7N:M+* = 93
Thc peak at m/z = 93 was chosen as thc molecular ion, because it is the
tallest peak in the cluster and it has plausible isotope peaks at M+l and M+2.
The significant peak at M-l could be from loss of 1 H. Thc tiny stuff at M2 and M-3 could be noise or, possibly, loss of more than I H.
With an odd mass, the nitrogen rule tells us that there are an odd number of
N atoms in the molecule.
rings + double bonds = c - A/2 + nJ2 + 1 = 6 - 7 / 2 + 1 / 2 + 1 = 4
Expected intensity of M+l is 1.08(6) +0.012(7)+ 0.369(1) - 6.93%
carbon
hydrogen
nitrogen
Observed intensity of M+1 = 71/999 = 7.1 %
Expected intensity of M+2 = 0.005 8(6)(5) = 0.17%
Observed intensity of M+2 - 2/999 = 0.2%
C2H6Hg: M+* = 228
(d) (CH3)2Hg
There are six strong peaks in an unfamiliar pattern. Given that only
elements from Tabic 21-1 are admissible, we notice that Hg has six
significant isotopes. By convention, wc take the lightest isotope, ,98 Hg, for
the molecular ion at m/z = 228. This leaves just 30 Da for thc rest of the
Chapter 21
molecule, which could be composed of two methyl groups.
In computing rings + double bonds, we include Hg as a Group 6 atom (like
O or S) because it makes 2 bonds.
rings + double bonds = c - A/2 + w/2 + 1 = 2 - 6 / 2 + 1 = 0 .
The peak at m/z = 228 is M+" = (CH3)2198Hg.
Small peaks at m/z = 227 and 226 could arise from loss of one or two H
atoms. If (CIl3) 2 l98 Hg loses H atoms, then all thc species at higher mass,
such as (CH 3 )2 ,99 Hg, will also lose H atoms. That is, each isotopic
molecule is going to contribute some intensity to peaks of lower mass. It
makes no sense for us to get too carried away with the analysis of thc
isotopic pattern, because each peak derives intensity from peaks at lower and
higher mass.
The peak at m/z = 229 is M+l, composed mainly of (CH3) 2 l99 Hg, with some
(12CH3)(l3CH3)198Hg + l 2 C 2 H 5 2Hl 98 Hg. Just considering Hg, the
predicted intensity, based on M+, is -9^7 x 100 = 169.2% of M + \ The
observed intensity is 215/130 = 165% of M+*. In this calculation, the
fraction -gijy- is the ratio of the abundances of 199Hg to l98 Hg. The peak at
m/z = 230 is M+2, composed mainly of (CH3)2200Hg. Thc predicted
23 10
isotopic intensity, based on M+, is ~^f
200
Hg
x 100 = 231.7% of M + \
Observed intensity of M+2 = 291/130 = 224% of M + \
Just considering Hg isotopes, we expect the peaks at M, M+l, M+2, M+3,
M+4, and M+6 to have the ratios 9.97 : 16.87 : 23,10 : 13.18 : 29.86 : 6.87
= 1 : 1.69 : 2.32 : 1.32 : 2.99 : 0.69.
Observed intensity ratio = 1 : 1.65 : 2.24 : 1.29 : 2.81 : 0.64.
(e) CH2Br2: M+* - 172
The three peaks at m/z • 172, 174 and 176, with approximate ratios 1 : 2 : 1
looks like the pattern from 2 Br atoms in Figure 21-7.
rings + double bonds = c-hl2 + nl2 +1 = 1 - 4 / 2 + 1 = 0
T
h includes H + Br
Expected intensity of M+l is 1.08(1) + 0.012(2) = 1.10%
carbon
hydrogen
Observed intensity of M+l = 12/531 = 2.3%. It is possible that this peak at
mlz = 173 also has contributions from CH^Bt^'Br. We have no way to
compute thc intensity at m/z = 173 if some of this peak comes from
275
Mass Spectrometry
CH79Br81Br. Given this ambiguity, we will just compare thc theoretical
pattern for 2 Br atoms to the observed pattern.
Theoretical intensity of M+2 = 97.3(2) - 194.6%
Observed intensity of M+2 = 999/531 = 188%
Theoretical intensity of M+4 - 47.3(2)(1) - 94.6%
Observed intensity of M+4 = 497/531 - 93.6%
(0
\
/-~\
7
l,10-Phenanthroline,Ci2H8N2: M+*=180
\=N
N=^
The strongest peak in the high-mass cluster is at m/z = 180, which could be
the molecular ion. It has plausible isotopic peaks at 181 and 182. Thc
significant peak at m/z = 179 could be from loss of 1 H.
I he intensity ratio M+1/M+* - 138/999 = 13.8%. We estimate that the
number of C atoms is 13.8/1.08 = 12.8.
If the molecule contains 13 C atoms, the formula might be C^HgO, which
would have 13 - 8/2 + 1 = 10ringsplus double bonds. The expected
intensity of M+l would be 1.08(13) +0.012 (8)+ 0.038(1) - 14.2%. The
expected intensity of M+2 would be 0.005 8(13)(12) + 0.205(1) = 1.1%.
Observed intensity of M+2 = 9/999 = 0.9%. The formula C^HgO fits the
data and a conceivable structure is
OOQ
O
If thc molecule contains 12 C atoms, the formula might be C12H4O2, which
would have 12-4/2 + 1 = 11ringsplus double bonds. A molecule with this
many rings + double bonds would be pretty implausible.
If the molecule contains nitrogen, it must contain an even number of N
atoms because the molecule has an even mass. A possible formula is
C|2HgN2, which would have 12 - 8/2 + 2/2 + 1 = 10ringsplus double
bonds. This turns out to be the correct formula, and the structure is shown at
the beginning of this answer. The predicted intensity of M+l is 1.08(12) +
0.012(8) + 0.369(2) = 13.8%, which is exactly equal to the observed
intensity. The expected intensity of M+2 is 0.005 8(12)(11) = 0.8%.
Observed intensity • 0.9%.
(g) C 3 ~
F e
Ferrocene, CioHioFc: M + *=186
~ £ ^
The strongest peak at high mass is at m/z = 186, which could be thc
molecular ion. It has plausible isotopic peaks at 187 and 188. Significant
peaks at m/z = 184 and 185 could be from loss of H. Calling M+* = 186, we
find the following ratios of peak intensities:
M-2
8.3
M-l
1.6
M +<
100
M+l
132
M+2
1.0
From the intensity ratio M+1/M+' = 13.2%, we could estimate that the
number of C atoms Is 13.8/1.08 = 12.8. From this we could propose
formulas like C13H1.4O or C12H10O2.
Alternatively, noting the significant intensity of M-2, we could propose that
the molecule has Fc in it, which, in fact, it does. For thc formula CioHjoFe,
we predict that M-2 will have an intensity of «jy^; x JOO = 6.37% of M + \
which is not terribly far from the observed value of 8.3%. The intensity at
M+l will have a contribution from 57 Fe and from
l3
C and 2 H. The 57 Fe
contribution is 2.119/91.754 = 2.31% of M+*. The other contributions are
1.08(10) + 0.012(10) = 10.92%. The total intensity predicted at M+l is
13.23% and the observed intensity is 13.2%. The predicted intensity
at M+2
J
• 0-282
1S
9L754 x
,-
1tAA
0
° (from Fc) + 0.005 8(10)(9) (from C) = 0.83%, and the
observed intensity is 1,0%.
The compound is dibromochloromethane:
212
CH8lBr237Cl
210
CH 8 'Br 2 35 CI + CH 79 Br 81 Br 37 Cl
208
CH 79 Br 8 'Br 3 5Cl + CH 79 Br 2 37 Cl
206
CH79Br235Cl
175
CH 81 Br 2
173
CH 79 Br 8 'Br
171
CH79Br2
8
162
iBr2
160
^BrS'Br
79
158
Br 2
131
CH 81 Br 37 Cl
129
CH8'Br35Cl + CH79Br37CI
127
CH 79 Br 3 5d
94
93
92
91
gl
79
50
49
43
47
37
35
CH 8 lßr
C 8 lßr
CH 79 Br
c 7 9 Br
81Br
79 Br
CH37C1
c 37 CI
CH^CI
C35C1
37C1
35 C1
277
Mass Spectrometry
21-15.
Thc CO2 that we exhale is derived from oxidation of the food wc eat. Thc chart
shows that the group of plants called C3 plants has less 13C than the groups
called C4 and CAM plants. If the diet in the United States contains more C4 and
CAM plants and thc diet in Europe contains more C3 plants, then thc difference
in 13 C content of exhaled CO2 might be explained.
21-16.
(a) Mass of proton + electron = 1.007 276 467 + 0.000 548 580
=1.007 825 047 Da. To thc number of significant digits in Table 1, thc
masses of the proton and electron are equal to the mass of ' H.
(b) mass of proton + neutron + electron
- 1.007 276 467 + 1.008 664 916 + 0.000 548 580 = 2.016 489 963 Da
mass of 2H in table = 2.014 10 Da.
The 2 H atom is 0.002 39 Da lighter than the sum of its elementary particles.
(c) Mass difference = (0.002 39 Da) (1.660 5 x IO' 27 kg/Da)
= 3.97 x IO' 30 kg
E = mc2 = (3.97 x 1 0 3 0 kg)(2.997 9 x IO8 m/s)2 = 3.57* 10"13 J
mc2 is the binding energy ofa single nucleus. For a mole, the energy is
(3.57x10-13 J)(6.022xl023 mol"1) = 2.15 x 1011 J/mol = 2.15 x 108kJ/mol.
(d) Binding energy for atom = (13.6 eV)(1.602 18 * 10-»9 J/eV) = 2.18 x ]0-l 8 J
To convert to a mole: (2.18 x I O ' 8 J)(6.022 x lO 23 mol 1 ) = 1.31 x 106
J/mol = 1.31 x 103 kJ/mol. The ratio of the nuclear binding energy to the
electron binding energy is (2.15 x IO8 kJ/mol )/(l.31 x lO3 kJ/mol)
= 1.64 x 105.
w
21-17.
79
21-18.
28
nuclear binding energy
bond energy
v
^
kJ/mo,
k J / m o | ) = 5 x 10 5
8
Br abundance = a = 0.506 9
' Br abundance + b = 0.493 1
79
3
Abundance of CH Br 3 = A = 0.130 2 5
Abundance of CH 79 Br 2 81 Br = 3a2A = 0.380 lo
Abundance of CH 79 Br 81 Br 2 = 3ab2 = 0.369 7 5
Abundance of CH 8l Br 3 = A3 = 0.119 9 0
Relative abundances: M + : M+l : M+2 : M+3 = 0.342 7 : 1 : 0.972 8 : 0.315 4
Si abundance = a = 0.922 30 29 Si = b = 0.046 83 3°Si = c = 0.030 87
(a + b + cp - a 3 + 3a2A + 3ii2c + 3aA2 + babe + 3ac 2 + A3 + 3A^ + 3Ac2 + c 3
278
Chapter 21
A
1
2
3
4
5
6
7
8
9
10
II
12
13
H
15
16
17
18
19
20
21
22
23
24
25
26
Silicon
a=
B
C
D
aA3 =
Relative
Composition
abundance
0.92230
0.784543
1.000000 28SÍ 28Si 28 Si
3aA2b =
b(mass = 84)
0.04683
0.119506
0.152326 28SÍ 28SÍ 29 Si
i:
3aA2c (mass = 85)
0.03087
0.078778
0.100412 28Si 28Si 30 Si
3abA2 (mass = 86)
0.006068
0.007734 28Si 29Si 29 Si
6abc •
(mass = 86)
0.008000
0.010197 28SÍ 29SÍ 30 Si
3acA2 (mass - 87)
0.002637
0.003361 28SÍ 30Si 30 Si
bA3
(mass - 88)
0.000103
0.000131 29Si 29SÍ 29 Si
Jb*2c
(mass = 87)
0.000203
0.000259 29SÍ 29SÍ 30 Si
3bcA2=
(mass = 88)
0.000134
0.000171 29Si 30Si 30 Si
cA3 =
(mass = 89)
2.94I8C-05
0.000037 30Si 30SÍ 30 Si
(mass = 90)
Check: sum of terms in column B =
1
mass:
intensity:
84
I
85
86
87
0.152 3 0.108 1 0.010 33
88
89
90
0.003 62 0.000 171 0.000 037
21-19.
In a double-focusing mass spectrometer, ions ejected from the source pass
through an electrostatic sector that selects ions with a narrow band of kinetic
energies to continue into the magnetic sector. The electric sector acts as an
energy filter and the magnetic sector acts as a momentum filter.
21-20.
From Box 21-2, we know that an ion of m/z = 500 accelerated through a potential
difference of V volts attains a velocity ofyj2zeV/m.
We need to express the mass
in kg. Thc footnote of Table 21-1 gives the conversion factor.
500 Da x 1.661 x io- 27 kg/Da = 8.30 x 1Q-25 k g
velocity =
\2zeV _
V m
= 4.39 x 10" m/s
/2(l)(1.602x 10-'9Ç)(5.00 \ lQ3y)
\j
8.30 x l0- 2 *kg
Mass Spectrometry
279
To figure out the units, remember that work (joules) - E'q = volts-coulombs. So
the product C x V = J = m2kg/s2. Putting these units into the square root gives
velocity in m/s.
The time needed to travel 2.00 m is (2.00 m)/(4.39 x IO4 m/s) = 45.6 ps. If we
repeated a cycle each time this heaviest ion reaches thc detector, we could collect
1/(45.6 ps) • 2.20 x 104 spectra per second.
If we double the mass in the square root to get up to 1 000 Da, the velocity
decreases by 1^2 and the frequency goes down by \y¡2 to 1.56 x IO4 spectra per
second.
21-21.
21-22.
The reflectron improves resolving power by ensuring that all ions of thc same
mass reach the detector grid at thc same time. Ions from the ion source have
some spread of kinetic energy. Faster ions penetrate deeper into the reflectron
and therefore spend more time there before being turned around. Thc reflectron
essentially allows slower ions to catch up to faster ions.
(a)
kT
_ (1.38xlQ- 2 3j/K)(300K) =
* " (y¡2cP) - (>/2<ll(10-9m)2)(10-5pa)) "
(The answer is in meters if you substitute m 2 -kgs 2 for J and kgm-'-s"2 for
Pa from Tabic 1-2.)
(b)
21 -23.
kT
_ (1.38 x 10-23 J/KX3Q0K)
*" " (-fiaP) ~ (V2(K(10-9rn)2)(10-8Pa))
Ions seen in elcctrospray usually existed in solution prior to electrospray.
Atmospheric pressure chemical ionization creates ions in the corona discharge
around thc high voltage needle.
21-24.
In collisionally activated dissociation, ions are accelerated through an electric
field and directed into a region with a significant pressure of gas molecules.
Collisions transfer enough energy to break molecules into fragments.
Collisionally activated dissociation can be conducted "up front" at the entrance to
the mass separator, or in the middle section (the collision cell) in tandem mass
spectrometry.
21-25.
A reconstructed total ion chromatogram shows the current from all ions above a
selected mass displayed as a function of time. The chromatogram is
"reconstructed" by summing the intensities for all observed values of m/z. The
total ion chromatogram shows everything coming off thc column. An extracted
280
Chapter 21
ion chromatogram displays detector current for just one or a few values of m/z as
a function of time. The intensity displayed is extracted from thc full mass
spectrum recorded at each time interval. A selected ion chromatogram also
displays detector current for just one or a small number of m/z values. However,
for a selected ion chromatogram, the detector is not measuring thc signal for all
values of m/z in each time interval. The detector is set at just thc desired values
of m/z and collects that information for the whole time. The extracted ion
chromatogram and the selected ion chromatogram are selective for an analyte of
interest (plus anything else that gives a signal at thc same m/z). The selected ion
chromatogram has improved signal-to-noise ratio because the most time is spent
detecting signal at the selected mass.
21-26.
In selected reaction monitoring, an ion of one m/z value is selected by the first
mass separator. This ion is directed to a collision cell in which it undergoes
collisionally activated dissociation to produce fragment ions. One of those
fragment ions is then selected by a second mass separator and passed through to
the detector. The detector is just responding to one product ion from the selected
precursor ion. This technique is called MS/MS because it involves two
consecutive mass separation steps. The signal/noise ratio is improved because
thc noise level is very low. There are few sources of the precursor ion other than
thc desired analyte, and it is very unlikely that other precursor ions of the selected
m/z can decompose to give the same product ion selected by the second mass
separator.
21-27.
(a) Ibuprofen can readily dissociate to form a carboxylate anion, so I would
choose the negative ion mode. It would be harder to form a cation.
The carboxylate anion should exist in neutral solution, since pKa is probably
around 4. In sufficiently acidic solution, the carboxylatc will be protonated.
I would use a neutral chromatography solvent to ensure a good supply of
analyte anions.
281
Mass Spectrometry
(b) The formula of the molecular ion, M", is C| 3 H, 7 0¿ . The intensity expected
at M+l is 1.08(13)+ 0.012(17)+ 0.038(2) = 14.32.
carbon
21-28.
hydrogen
oxygen
The analysis follows thc same steps as Table 21-3. The work is set out in the
following table. Peaks A and B give MA = 12 and peaks H and I give OH = 19.
The combination of peaks G and H give no = 21, which makes no sense and will
be ignored. Assigning peaks A, B, C... as n = 12,13, 14... gives the sensible,
constant molecular masses in the last column of the table. The mean value,
disregarding peak G, is 15 126.
Charge = n =
Observed m/z
Peak
A
M
C
D
E
F
G
H
1
21-29.
21-30.
BjWfl
m , , + l - 1.008
f»n-mn+\
1 163.6
96.9
—
—
—
—
—
—
—
—
—
—
—
—
—
-—
796.1
756.2
37.2
39.9
1 261.5
I 164.6
834.3
797.1
757.2
OT„.M-1.008
12.0i~12
[13]
[14]
[15]
[16]
[17]
21.4 [18]
18.95-19
[20]
Molecular mass
= «x(m„-1.008)
15 126
15 127
—
—
—
—
14 999
15 126
15 124
mean = 15 100
mean without peak G = 15 126
Thc separation between adjacent peaks is 0.27,0.28, 0.25, 0.24,0.24, 0.24, 0.27,
0.23,0.24,0.25,0.26, and 0.24 m/z units, giving a mean value of 0.251. If
species differing by 1 Da are separated by 0.251 m/z unit, the species must carry 4
charges (z = 4). The mass of the tallest peak must be 4(1 962.12) = 7 848.48 Da.
Expected intensities for 37:3, whose formula is [MNH4]+ = C37H72ON
X + 1 = 0.012/iH + 1.08»c + 0.369WN + 0.038«o
= 0.012(72) + 1.08(37) + 0.369(1) + 0.038(1) = 41.2% (observed = 35.8%)
X + 2 = 0.005 8«c("c-l ) + 0.205*0
= 0.005 8(37)(36) + 0.205(1) = 7.9% (observed = 7.0%)
Expected intensities for 37:3, whose formula is [MH]+ = C37H69O
X + 1 =0.012KH + 108«c + 0.038«o
282
Chapter 21
= 0.012(69) + 1.08(37) + 0.038(1) = 40.8% (observed = 23.0%)
X + 2 = 0.005 SnC(nç-l) + 0.205« o
= 0.005 8(37)(36) + 0.205(1) = 7.9% (observed - 8.0%)
Expected intensities for 37:2, whose formula is [MNH4]+ = C37H74ON
X + 1 = O.OI2nH + 1.08wc + 0.369/TN + 0.038no
- 0.012(74) + 1.08(37) + 0.369(1) + 0.038(1) - 41.3% (observed - 40.8%)
X + 2 = 0.005 8nC(/7c-l) + 0.205*0
= 0.005 8(37)(36) + 0.205(1) = 7.9% (observed = 3.7%)
Expected intensities for 37:2, whose formula is [MH]+ = C37H 71 0
X + 1 = 0 . 0 1 2 « H + 108n c + 0.369nN + 0.038« o
= 0.012(71) + 1.08(37) + 0.038(1) = 40.8% (observed = 33.4%)
X + 2 = 0.005 Snc(nc - 1) + 0.205/iO
= 0.005 8(37)(36) + 0.205(1) - 7.9% (observed = 8.4%)
21-31.
Selected reaction monitoring chooses the molecular ion CIO3 (m/z - 83) with the
mass separator Ql. In collision cell Q2, this species could possibly undergo thc
following decomposition:
35C10J
" ¡ Ä
HCIOä
+
35
C10-
+
»Ct
collisions
m/z = 83
miz = 67
m/z = 5l
miz = 35
Quadrupole Q3 selects only m/z = 67. The measurement is specific for CIO3
because there are probably few compounds in water producing ions at mlz = 83,
and very few of them are likely to decompose into m/z = 67. None of the species
C10¿, BrOj, or IO] can produce m/z = 83 to be selected by Ql.
21-32.
(a) Consider thc term AxCxmx, which applies to the unknown:
AxCx™x
=
(
pmol isotope A
^ ( pmol V ^
~ Ijimol isotope A + pmol isotope BJ [g unknownj fe u n k n o w n )
(
pmol isotope A
\
l^imol isotope A + pmol isotope BJ (M-mo1 v )
(
umol isotope A
>
=
\iimo\ isotope A + pmol isotope B) ^ m o 1 i s o t o P c A + pmol isotope B)
= pmol isotope A in the unknown.
Similarly, BxCxmx = pmol isotope B in the unknown, AsCsmsx = pmol
isotope A in the spike, and BsCsms = pmol isotope B in the unknown.
When we mix the unknown and the spike, thc isotope ratio is
283
Mass Spectrometry
pmol A pmol A in unknown - pmol A in spike
R = pmol B : : pmol B in unknown + pmol B in spike
AxCxmx + ¿sCV"s
B\Cxmx + BsCsms *
(b) Cross-multiplying Equation A gives
R(BxCxmx + BsCsms) = AxCxmx + AsCsms
RBxCxmx + RBsCsPis = AxCxmx + AsCsms
RBKCxmx-AxCxmx = AsCsPts- RBsCsms
AsCsms — RBsCsms
RBxmx - Axmx
(Csms) (AS-RBA
Cx =
z
[mx
){RBX-AX)
51
5
(c) A = VandB = °V
Atom fractions in unknown. Ax = 0.997 5 and Bx = 0.002 5
Atom fractions in spike: As = 0.6391 and fis = 0.360 9
(Qm^ (AS-RBS
C« - m ){RB -A
K
X X
fT2.243 5umolV/g)(0.41946gV) f 0.639 1 -(10.545X0.3609)^
=
I
J l(10.545)(0.002 5) - 0.997 5J
0.401 67 g
= 7.639 4 pmol V/g
,Ä „
f(2.243 5 umol V/gj(0.41946 g)W0 6391 -(10.545X0.3609)
(d) Cx - ^
0.401 67 g
AC10.545X0.0025-0.997 5)
((2.243 5 umol V/gX0.41946 gft f_0-639 1 - 3.8057
"I
0.401 67 g
){0>.0263o -0.997 5
f-3.16^
=
23429
(
)[lÔ97iTj
= 7.639 pmol V/g
V
CHAPTER 22
INTRODUCTION TO ANALYTICAL SEPARATIONS
22-1.
Three extractions with 100 mL are more effective than one extraction with 300
mL.
22-2.
Adjust the pH to 3 so the acid is in its neutral form (CH3CO2H), rather than its
anionic form (CH3CO¿).
22-3.
(a) The EDTA complex is anionic (AIY"), whereas the 8-hydroxyquinolinc
complex is neutral (AIL3),
(b) The EDTA complex is anionic (AIY), so wc need a hydrophobic cation such
as (C s H| 7 )iNlf to try to bring hydrophobic AIY" into the organic solvent.
22-4,
Thc complcxation reaction mHL + Mm+ F* MLm + mH + is driven to the right
at high pH by consumption of H+. This consumption increases the fraction of
metal in the form MLm, which is extracted into organic solvent.
22-5.
Thc form that is extracted into organic solvent is ML„. The formation of ML,, is
favored by increasing the formation constant (ß). ML„ is also favored by
increasing Ka, which increases the fraction of ligand in the form L". Increasing
Ki decreases thc fraction of ligand in the aqueous phase, thereby decreasing the
formation of ML„. Increasing [H+] decreases the concentration of L" available
for complexation.
22-6.
When pH > pKm+, thc predominant form is B, which is extracted into the
organic phase. WhcnpH > pATa for HA, thc predominant form is A", which is
extracted into thc aqueous phase.
22-7.
K
(a) S H2 o SCHCI3
= [S]CHCI 3 /[S]H 2 0 = 4.0
[S]CHCI3 - ATS]H2o - (4.0X0.020 M) = 0.080 M
{0)
mol S in CHCI3
mol S in H 2 0
(0.080 MX 10.0 mL)
(0.020 M)(80.0 mL) ~ °- 5 0
22-8.
Fraction remaining = { ^ ^
22-9.
(a) D =
- (gQ.o^OXlO.O)) 6 = 0.088
[BJc6H6
[B]H 2 O+[BH+]H 2 O
284
285
Introduction to Analytical Separations
(b) D is the quotient of total concentrations in the phases.
K is the quotient of concentrations of neutral species (B) in the phases.
K-Ka
(c)
D
=
(50.0yi.Qx lQ-9)
+
Ka+[H )
"
(1.0 x IO"9) + (1.0 x IO-8)
(d) D will be greater at pH 10 because a greater fraction of B is neutral.
[MLff]Qrg
22-10.
From Equation 22-12, D ~ r_M/t+i
Ejyfia
^extraction [H + ]£,
_
Comparing this result to Equation 22-13 gives Attraction
Constant
KM
=
KMK
w
Reason
Effect on Attraction
.
increase
ML„ is more soluble in organic phase,
ß
increase
Ligand binds metal more tightly and
ML„ is the organic-soluble form.
K
increase
Ligand dissociates to L" more easily,
increasing ML« formation.
KL
decrease
HL is more soluble in organic phase, where
it is not available to react with
22-11.
(a) D = K[H+]/([H+] + *a) = 3-10^»/(10^00 +
L52
M^aq).
x 10"5) = 2.60 at P H 4.00.
Fraction remaining in water = q = Vxi(V\+DV2) = 100/[ 100+2.60(25)] =
0.606. Therefore, the molarity in water is 0.606 (0.10 M) = 0.060 6 M.
The total moles of solute in the system is (0.100 L)(0.10 M) - 0.010 mol.
The fraction of solute in benzene is 0.394, so the molarity in benzene is
0.394 (0.010 moiyO.025 L = 0.16 M.
(b) AtpH 10.0: D = 1.97 « 10"5, q - 0.999995 1, molarity in water =
0.10 M, and molarity in benzene = 2 x IO"6 M.
22-12.
22-13.
D - C/[H+]", where C = K M ^ K ^ ^ ^ L
D\ - 0.01 = C/[H +]2 andD 2 = 100 = C/fH+g
ZtyD, = IO4 =[H + ]?/[H + ]? => [H + h/[H + ] 2 = 102
0
ApH = 2pHunits
(a) Since there is so much more dithizone than Cu, it is safe to say that [HL] org
= 0.1 mM.
D=
KMK
~lq~
[HL]o"rK
[H^
=
(7xlQ 4 X5x 1022X3x10-5)2 (1 * 1Q-4)2
(1.1 x 104)2
[H+p
286
Chapter 22
- 2.6 x HHatpH 1 and 2.6 x 10'<>atpH4
0>) q = V\f(V\+DV2) = 100/[100 + 2.6x 104(10)] = 3.8 x IO-4
22-14.
(a) D =
[ML2]or£ _ c o r K r o r e
[ML2]laq
Caq ^aq
=
% extracted -
r
Lorg - V C a q
3
100 Dp
K
M
100C org
_|00DQq
^+ " ^ =
*-aq
j ^
Corg
Caq + D Caq
^
2
y
,,
«g
1+D
** forg
org
(b) Spreadsheet for pH dependence of dithizone extraction
H
K(M) =
D
pH
H
70000
Beta =
5E+I8
Ka =
0.00003
Kiti
10
K
12
13
14
(1.01)001
v(«q)°
100
15
16
2.60E+00
3.98E-03
2.5IE-03
4.13E+01
2.8
I.58E-03
1.04E+02
I.OOE-03
6.3IE-04
3.98E-04
2.51E-04
1.58E-04
IOOE-04
1.00E-05
2.60E+02
3.2
3.4
3.6
3.8
V(°K) =
1.00E-02
6.31E-03
2.2
2.4
2.6
11000
IHL]org =
i:
D = Dist.coeff % extracted
I.OOE-OI
2.60E-02
0.05
6.54E+00
I.64E+01
6.54E+02
I.64E+03
4.I3E+03
1.04E+04
2.60E+04
2.60E+06
4.95
11.57
24.73
45.21
67.46
83.89
92.90
98.80
99.52
99.81
100.00
C2=10 A -B2
17 D2 = (ÍA$2«SAS4»$AS6A2*$AS10A2y(SA$8*2«C2A21
18 IE2-(D2«$AS12/$A$14V(I+(D2*$A$12/SA$14^*100
9
m f
1
287
Introduction to Analytical Separations
22-15.
D
E
A
B
C
1 Liquid-liquid extraction efficiency
2
50 mL (volume of extraction solvent)
3
v2 =
50 mL (volume to be extracted)
4
v,K =
2 (partition coefficient = [SMS],)
5
F
6 Divide V2 into n equal portions for n extractions
7 Theoretical maximum fraction extracted • 1-qm,it = l-expirvyVJK)
0.864665
8
1-<W =
9
V2/n
10
i
i
individual
q=
1-q=
% of limiting
11
extraction fraction
fraction
fraction
12
n
volume remaining extracted extracted
13
1
50.0
0.333
0.667
77.1
14
2
25.0
0.250
0.750
86.7
15
0.216
0.784
90.7
3
16.7
16
4
12.5
0.198
0.802
92.8
17
5
10.0
0.186
0.814
94.1
18
6
8.3
0.178
0.822
95.1
19
7
7.1
0.172
0.828
95.7
20
8
6.3
0.168
0.832
96.2
21
9
5.6
0.164
0.836
96.6
22
10
5.0
0.162
0.838
97.0
23
24 C14= ($B$4/($B$4+B14*$B$5)rA14
25 q = [Vi/ry, + (V2/n)K)*n
The theoretical limit for fraction extracted is in cell C8. 95% of thc theoretical
fraction extracted is (0.95)(0.864 6) - 0.821 4. This fraction is exceeded with n
6 equal extractions.
1.0
0.9
0.8
"8 0.7
o
e 0.6
« 0.5
' 0.4
0.3
0.2
0.1
0.0
I-
2
10
288
Chapter 22
22-16.
1-C, 2-D, 3-A, 4-E, 5-B
22-17.
The larger thc partition coefficient, the greater the fraction of solute in the
stationary phase, and the smaller thc fraction that is moving through the column.
22 IS
{ \ k = tímc s o l "te spends in stationary phase
^ '
time solute spends in mobile phase
U- *m
fa
=
h_
tm
(b) Fraction of time in mobile phase = ; . . = . _"\ • = -r—y
K
tm + ts
tm + tom 1 + A
(c) Ä • 7 " •
(
+t
= TT¿- Parts (b) and (c) together tell us that
time for solvent to pass through column
time for solute to pass through column
22-19.
time spent by solute in mobile phase
total time on column
(0 461 cm"\2
(a) Volume per cm of length = 7rr2 x length = n I — j
1 (1 cm) = 0.167 mL
mobile phase volume = (0.390)(0.167 mL) = 0.065 1 mL per cm of column
_
1.13 ml/min
..
linear flow rate = ux = 0 .065 1 mL/cm " 17.4cm/min
(b) / m = (I0.3cm)/(17.4cm/min) = 0.592 min
(c) k = ——
=> tx = ktm + tm = 10(0.592)+ 0.592 = 6.51 min
'm
22-20.
(a) Linear flow rate = (30.1 m)/(2.16 min) = 13.9m/min.
Inner diameter of open tube = 530 pm - 2(3.1 pm) = 523.8 pm
=> radius = 261.9 pm.
Volume = Ttr2 x length = n (261.9 x 10"4 cm)2(30.1 x io 2 cm) = 6.49 mL
Volume flow rate = (6.49 mL)/(2.16 min) = 3.00mL/min
tws t
(b) k =
'r~'m
~hT=
17.32-2.16
2.16
„
M
=702
A = tsltm (where / s • time in stationary phase)
r*
•
*> +
S
"•''ill
traction oftimc in stationary phase = . . . = ,. , — =
's "*" 'm
*'m + <m
k _ 7.02
k+\
7.02+ 1 " ° ' 8 7 5
(c) Volume of coating » 2nr x thickness x length
= 2ii[(261.9+1.55)x 10^cm](3.1 x HHcmX30.1 x IO2 cm) = 0.154 mL
289
Introduction to Analytical Separations
22-21.
Large load _ fLarge column radiusV
(a) Small load : Umall column radiusj
100 mg
fLargc column diameter^2
.
..
6
-TT— = —:rV;
jj
:
=> arge column diameter = 4.25 cm
6
V 0.85 cm diameter J
4.0 mg
Use a 40-cm-long column with a diameter near 4.25 cm.
(b) The linear flow rate should be the same. Since thc cross-sectional area of thc
column is increased by a factor of 25, the volume flow rate should be
increased by a factor of 25 => wv = 5.5 mL/min.
(c) Volume of small column - nr2 x length = TI(0.85/2 cm)2(40 cm) = 22.7 mL
Mobile phase volume = 35% of column volume = 7.94 mL
Linear flow =
22-22.
(7.94
m L y f o S mL/min)
=
l 11 c m / m i n f o r b o t h c o l u m n s
•
90 30
' 3 Q " = 2.0
(a) k =
(b) Fraction of time solute is in mobile phase = ~ = Q"Q = 0.33
22-23.
A volume flow rate of 0.20 mL/min corresponds to a linear flow rate of
Solvent volume per cm of column length = ( 0 . 1 5 ) i i f - ^ — J = 0.0106 mL/cm.
' 0.20 mL/min > . .
.
É0.0106 mL/cmJ
22-24. h = Ky--
v
=19cnVm,n
3© = |
m
ForAT=30,A = 3oQ = 6.
16.6
K = k~¡r = (3-59)T¿7 -
22-26.
K=
k =
-
4.69
Vm
ky
/r^m
tm
433-63 _
=
63
~5-87
290
Chapter 22
Vm m
-K-r2 x 4engthVi " 2-fírr x thickness x 4ength-
=
(103)2
2(103.25) x 0.5
=!
1028
(In the numerator, r refers to the radius of the open tube = l/2 (207 - 1.0) pm
= 103 pm. In thc denominator, r is thc radius at the center of the stationary
phase, which is 103 + 2 (0.5) = 103.25 pm.)
y
Therefore, the partition coefficient is A" = ky~ = 5.87(102.8) • 603.
t
iré
m.
Fraction of time in stationary phase = . , , = ,, "V = 7-—-r
} v
*&+tm
ktm + tm
k+ 1
5 87
22-27.
(a) After 10 cycles, the compounds have passed through a length 10Z, containing
ION theoretical plates. We are told that y - 1.018.
resolution = "4 ( 7 - I )
1.60 - ^ p ( 1 . 0 1 8 - 1) => N = 1.26 x 104
(b) Plate height = H = UN = 50 cm/1.26 x IO4 = 4.0 x 10-3cm = 40pm
(c) Resolution is proportional to s[Ñ or ^/number of cycles
resolution in 2 cycles
[~2
resolution in 10 cycles
\ll0~
^A41
resolution in 2 cycles • 0.447(resolution in 10 cycles) = 0.447(1.6) = 0.72
(observed resolution = 0.71)
22-28.
(a) Column 1 (sharper peaks)
(b) Column 2 (large plate height means fewer plates means broader peaks)
(c) Column 1 (less overlap between peaks because they are sharper)
(d) Neither (relative retention (= t^B)IT¿A)) is equal for the two columns
(e) Compound B (longer retention time)
(f) Compound B (longer retention time means greater affinity for stationary
phase)
(g) Y B V A = 10/8 =1.25
22-29.
Thc linear rate at which solution goes past the stationary phase determines how
completely the equilibrium between the two phases is established. This
Introduction to Analytical Separations
291
determines the size of the mass transfer term (Cux) in the van Deemter equation.
The extent of longitudinal diffusion depends on thc time spent on thc column,
which is inversely proportional to linear flow rate.
22-30.
Smaller plate height gives less band spreading: 0.1 mm
22-31.
Diffusion coefficients of gases are IO4 times greater than those of liquids.
Therefore, longitudinal diffusion occurs much faster in gas chromatography than
in liquid chromatography.
22-32.
The smaller the particle size, the more rapid is equilibration between mobile and
stationary phases.
22-33.
Minimum plate height is at 33 mL/min.
22-34.
Silanization caps hydroxyl groups to which strong hydrogen bonding can occur.
22-35.
Isotherms and band shapes are given in Figure 22-21. In overloading, the solute
becomes more soluble in the stationary phase as solute concentration increases.
This leaves little solute trailing behind the main band, and gives a non-Gaussian
shape. Tailing occurs when small quantities of solute arcretainedmore strongly
than large quantities. The beginning of thc band is abrupt, but the back part trails
off slowly as the tightly bound solute is gradually eluted.
22-36.
With 5.0 mg, thc column may be overloaded. That is, the quantity of solute per
unit length may be too great for the volume of stationary phase. This leads to the
upper nonlinear isotherm in Figure 22-21, which broadens bands and decreases
resolution.
22-37.
Equation 22-26 says that the standard deviation of the band is proportional to \jt.
Here is what we know of the rate of diffusion:
time
standard deviation
t\
o"i = 1
t2 = t\ + 20
<J2 - 2
/3 = t\ + 40
03 = ?
From thc bandwidths at times t\ and t2, we can write
292
Chapter 22
For time ty. ^
<»i -to
22-38.
= ^
=> f
= ^
/ ^ -
^
= 2 .65 mm
CT3
5 55 f
/ x u
- r
5.55 (9.0 min)2
(a) AT- - ^ =
^ ^
> = i., 2 x o p i a t e s
(b) (10cm)/(l.l 2 x IO2 plates) = 0.89 mm
i-> io
22-39.
,n\ u
(a) N =
4ÏJ
WW0.\)2
(A/B)+1>25
41.7 (900 s/44s>2
= ( 3 3 s/11 s) +(1.25)
= 4 1
* «^P'»**
(b) To use the equation N = (/¿a) 2 , we need to find the standard deviation of thc
peak. The width at 1/10 height is 22 + 22 = 44 s, which we are told is equal
to 4.297a. Therefore, a = (44 s)/4.297 = 10.24 s. N = (trlày2 =
(900 s/10.24 s) 2 = 7.72 x 103 plates.
The equation for an asymmetric peak from (a) gives
41.7(r/wQ,i)2
41.7 (900 s/44s) 2
N =
(A/B) +1.25 - (22 s/22 s) + (1.25) = 7 " 7 5 x
22-40.
.
10
Resolution = -£ = ^j-jjjj = 0.83. This is most like the diagram for a
resolution of 0.75.
22-41.
Since w = 4Vxly¡Ñ, w is proportional to Vr (if/v* is constant).
W2lwi = V2IV\ = 127/49 => w2 = (127/49)(4.0)= 10.4 mL.
™
Plalcs
-A" IW " i f ) 2 - 283.96 S2
^injection = (0-40 mL)/(0.66 mL/min) = 0.606 min = 36.36 s
2
^injection
injection =
12
36.362
~Ï2~
=
1I0
-19S2
A/detector • (0.25 mL)/(0.66 mL/min) = 22.73 s
CT
deLtor = (A0de?ector/12 = 43.04 S2
2
—
2
a
°obs " column
7
2
a
injcction
+ a
delector
283 9
- 6 = Column + 11019 + 43.04 =>
wi/2 - 2.35 a c o | u m n = 26.9 s
acolunin
- 11.4 3 s
293
Introduction to Analytical Separations
22-43.
''r2
h
K2
18
a = ^ = ^ = ^ = T5 = 1.20
h = K2J¡- = « ( p ) = 6.0
k] = 1 5 ( 3 ^ ) = 5.0
k\ = (t\ - trnVtm = t\ltm - 1 => '1 = >m(*i + O = (1-0 min)(5.0 + 1) = 6.0 min
k2 = (t2 - 'm)/'m
=> tl= tm(h + 1) = (1.0 min)(6.0 + 1) = 7.0 min
y = t2lt\ =7.0/6.0= 1.167
Resolution = 4 ( y - 1 )
1.5 = ^ ( 1 . 1 6 7 - 1 )
22-44.
=> 1.3 x 103 pi a t e s
(a) y = / 2 //| = 1.01, 1.05, or 1.10
V/V
(4 x resolution^
Resolution = 4 ( y - 1) => N = I
—j
I
resolution
2.0
2.0
2.00
N
640 000
Y
1.01
1.05
25 600
6 400
1.10
(b) For thc same kind of column, N can be increased by increasing the column
length (N oc -\/L ). y can be increased by changing solvent and/or stationary
phase to change the partition coefficients of the two components.
22-45.
(a) C 6 HF 5 : Í, = 12.98-1.06 = 11.92 min. k = 11.92/1.06 = 11.25
C 6 H 6 : t'r = 13.20-1.06 = 12.14min. k = 12.14/1.06 = 11.45
(b) a = 12.14/11.92 = 1.018
(c) y = /2//i = 13.20/12.98 =1.017
(d) W|72(C6HF5) = 0.124 min; wm (C 6 H 6 ) = 0.121 min
5.55 tT2 5.55(12.98)2
r
C 6 HF 5 : iV = - ^ 2 - =
= 6.08 x IO4 plates
0\242
P,atehei
*
ht
C6H6: N -
30.0 m
" 6.08 XIO 4 plates
555 ( 1 3
0)2
0 1 2 12
=
°493
mm
= 6.60 x IO4plates
294
Chapter 22
30.0 m
6.60 x IO4 plates = 0.455 mm
Plate height =
(e) w(C 6 HF 5 ) = 0.220 min; w(C6H6) - 0.239 min
2
»6/r2
16(12.98)
f
C 6 HF 5 : ¿V : - ^ = ' ^ 2 2 0 7
• 5.57 x IO4 plates
M
C6H6: N =
(f)
0)2
l6 ( 3
0 2 3^2
= 4.88 x IO4 plates
A/r
13 20 - 12 98
Resolution = — =
' 0.229 '
=
096
(g) N = V(5.57 x 104)(4.88 x io 4 ) = 5.21 x IO4 plates
_ *JE,
.. "N/5.21 x IO 4
Resolution =
(1.017- 1)= 0.97
(Y-l)
22-46.
Initial concentration (m) = 10 nmol/(l .96 * 10~3 m2) = 5.09 x 10-6 mol/m2.
Diffusion will be symmetric about the origin. Only diffusion in the positive
direction is computed below for / = 60 s. Other conditions in the graphs are
obtained by changing t and thc diffusion coefficient D.
A
1
2
3
4
5
6
7
C
Diffusion problem
x(m)
0
0.0001
0.0002
0.0003
0.0004
c(mol/m3)
4.637E-03
4.518E-03
4.178E-03
3.666E-03
3.057E-03
0.0005
0.0006
2.418E-03
1.816E-03
5.093E-06
0.0007
0.0008
1.294E-03
8.758E-04
1.600E-09
0.0009
0.001
0.0012
0.0014
0.0016
0.0018
0.002
5.625E-04
3.430E-04
1.090E-04
2.815E-05
5.901 E-06
1.0O4E-06
1.388E-07
moles =
1.00E-08
diameter (m) =
0.05
2
x-sectional area (m )
8
9
B
0.001963495
m (mol/m2)=
10
2
11 D (m /s) =
12
13 t(s) =
14
60
15
16
17
18 A10 = A4/A8
19
20 C3 = ($A$10/(SQRT(4*PI()*$A$12*$A$14)))
21
*EXP(-(B3*2)/(4*$A$12*$A$14))
295
Introduction to Analytical Separations
0.020
-1—r
0.005
1 min
| 0.004
o
.g
0)
ID
E
o
Methanol
diffusion
o 0.002
—
-tH•
i~
'
1_
o
0.000
0.008
10 min
b
10 min
-ö 0.004
o
.100 min
8
Ribonuclease
diffusion
s3 0.012
o
8 0.001
1 min
0.016
13
I 0.003
r^g
o
0.000
0
1 2
3
4
5
0.0
6
0.4
0.8
B
Plate height = HD + / 7 m a s s transfer = ~ + ( Q + C m ) %
2D,m
ux
2k&
+' h(k+\)2Ds
l + 6/t+llJfc2r2
24(k+\?Dm
"A
Parameters for 0.25 pm thick stationary phase
Ds= 1.0 x 10"9m2/s
A n = l . O x 10-5 m2/s
r=12.5 x l0- 4 m
¿ = 2 . 5 x 10-7 m
A= 10
0.0008
Stationary phase
thickness = 0.25 pm
0.0006
JE
a
0.0004
I
n
0.0002
Diffusion
n. n ooo
0.0
100 min
1.2
1.6
Distance (mm)
Distance (mm)
22-47.
-r—>—r
- — I — < -
0.2
0.4
0.6
Linear flow rate (m/s)
0.8
2.0
296
Chapter 22
0.0008
Stationary phase
thickness = 2 pm
0.0006
I
a? 0.0004
I
0 0OQ2
Diffusion
0.0000
0.0
0.2
0.4
0.6
0.8
1.0
Linear flow rate (m/s)
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
A
B
C
D
E
F
Plate height calculation for 0.25-um-thick stationary phase
H (mas s transfer)
|
Dm=
k(m/s) H (diffusion) C$ term Cm term
H (total)
0.00001
D5 =
1E-09
0.01
0.05
2.00E-03 3.44 E-08
4.00E-04 1.72E-07
6.25E-06
3.12E-05
2.01 E-03
4.31E-04
2.63E-04
2.26E-04
2.55E-04
3.01 E-04
3.54E-04
4.10E-04
4.68E-04
5.27E-04
5.88 E-04
6.48E-04
0.1 2.00E-O4 3.44E-07 6 25 E-05
k=
0.2 1.00E-04 6.89E-07
1.25E-04
10]
0.3 6.67E-05 1 03E-06 1 87E-04
0.4 5.00E-05 1.38E-06 2.50E-04
d (m) =
2.50E-07
0.5 4.00E-05 1.72E-06 3.12E-04
r (m) =
0.6 3.33E-05 2.07E-06 3.75E-04
1.25E-04
0.7 2.86E-05 2.41 E-06 4.37 E-04
0.8 2.50E-05 2.75 E-06 5.00E-04
0.9 2.22E-05 3.10E-06 5.62E-04
1 2.00E-O5 3.44E-06 6.25E-04
C4 = 2-$A £4/B4
D4 = 2*$A $8*$A$10A2*B4/(3*($A$8+1 ) A 21;A$6)
E4 = (1+6* $A$8+11*$A$8A2)*$/ $ 12 A2*B4/(24*($A$8+1 )A2*$A$4)
F4 = C4+D 4+E4
I
I
I
For stationary phase thickness = 0.25 pm, plate height contribution from mass
transfer in the stationary phase is negligible, as shown in the first graph. If the
stationary phase is 2.0 pm thick, plate height from mass transfer in the stationary
phase is not negligible, but it is still less than plate height from mass transfer in
the mobile phase. Cs and total plate height in the second graph are greater than in
the first graph. Cm and longitudinal diffusion terms arc unaffected.
297
Introduction to Analytical Separations
22-48.
Inspection of Equation 4-3 shows that the general form of a Gaussian curve is y ¿e-(x - xoßl2cs2f where /lisa constant proportional to thc area under the curve, x0
is thc abscissa of thc center of thc peak, and o is the standard deviation. We can
arbitrarily let a = 1, which means that the width at the base (w = 4a) is 4. A peak
with an area of 1 centered at the origin is y = 1 *e-<*)2/2. A curve of area 4 is y =
4*e-<x-jc0)2/2 The resolution is Ax/w. For a resolution of 0.5, Ax - 0.5*>v = 2.
That is, the second peak is centered at x = 2 if theresolutionis 0.5. Its equation
is y = 4*e-<*-2>2/2. Similarly, for aresolutionof 1, Ax = 1 * w = 4 and the second
peak is centered at x = 4. For a resolution of 2, the second peak is centered atx =
8. Thc equations of the curves plotted below are:
Resolution = 0.5:
Resolution = 1 :
Resolution = 2:
y = 1 »erW2« + Vt^x-W*
y = 1 *e<x)2/2 + 4*e<*^)212
y=\ *e~ix)2/2 + 4*e^- 8 ) 2 / 2
Resolution = 0.5
: r,
CD
Resolution = 1
Resolution = 2
i
\
:¿
S
?
re
il:
1
n 6 8 0 *
B^-O-Ó-0
J
y
Retention time (from center of first peak)
12
CHAPTER 23
GAS CHROMATOGRAPHY
23-1.
(a) Low boiling solutes are separated well at low temperature, and the retention
of high boiling solutes is reduced to a reasonable time at high temperature.
(b) Higher pressure gives higher flow rate. If pressure is increased during a
separation, retention times of late-eluting peaks are reduced. The effect is
the same as increasing temperature, but high temperatures are not required.
Pressure programming reduces the likelihood of decomposing thermally
sensitive compounds.
23-2.
(a) Packed columns offer high sample capacity, while open tubular columns
give better separation efficiency (smaller plate height), shorter analysis time,
and increased sensitivity to small quantities of analyte.
(b) Wall-coated: liquid stationary phase bonded to the wall of column
Support-coated: liquid stationary phase on solid support on wall of column
Porous-layer: solid stationary phase on wall of column
(c) Bonding or cross-linking the stationary phase reduces thc tendency for thc
stationary phase to bleed from the column during use.
23-3.
(a) Open tubular columns eliminate the multiple path term (A) from the van
Deemter equation, decreasing plate height. Also, the lower resistance to gas
flow allows longer columns to be used with the same elution time.
(b) Diffusion of solute in H2 and He is more rapid than in N 2 . Therefore,
equilibration of solute between mobile phase and stationary phase is faster.
23-4.
(a) Split injection is the ordinary mode for open tubular columns. It is best for
high concentrations of analyte, gas analysis, high resolution, and dirty
samples (with an adsorbent packing in thc injection liner). Splitless injection
is useful for trace analysis (dilute solutions) and for compounds with
moderate thermal stability. On-column injection is best for quantitative
analysis and for thermally sensitive solutes that might decompose during a
high-temperature injection.
(b) In solvent trapping, the initial column temperature is low enough to
condense solvent at thc beginning of the column. Solute is very soluble in
the solvent and is trapped in a narrow band at thc start of thc column. In
298
•
Gas Chromatography
¿yy
cold trapping, the initial column temperature is 150' lower than the boiling
points of solutes, which condense in a narrow band at the start of the
column. In both cases, elution occurs as the column temperature is raised.
23-5.
(a) All analytes
(b) Carbon atoms bearing hydrogen atoms
(c) Molecules with halogens, CN, NO2, conjugated C=0
(d) P and S and other elements selected by wavelength
(e) P and N (and also hydrocarbons)
(f) Aromatic and unsaturated compounds
(g) s
(h) Most elements (selected individually by wavelength)
(i) All analytes
23-6.
The thermal conductivity detector measures changes in thc thermal conductivity
of the gas stream exiting the column. Any substance other than the carrier gas
will change the conductivity of the gas stream. Therefore, thc detector responds
to all analytes. Thc flame ionization detector burns eluatc in an H2/O2flameto
create CH radicals from carbon atoms (except carbonyl and carboxyl carbons),
which then go on to be ionized to a small extent in the flame: CH + O - •
CHO+ + e". Most other kinds of molecules do not create ions in the flame and
are not detected.
23-7.
A reconstructed total ion chromatogram is created by summing all ion intensities
(above a selected value of m/z) in each mass spectrum at each time interval
during a chromatography experiment. The techniquerespondsto essentially
everything elutcd from the column and has no selectivity at all.
In selected ion monitoring, intensities at just one or a few values of m/z arc
plotted versus elution time. Only species with ions at those m/z values are
detected, so thc selectivity is much greater than that of the reconstructed total ion
chromatogram. The signal-to-noise ratio is increased because ions are collected
at each m/z for a longer time than would be allowed if the entire spectrum were
being scanned.
300
Chapter 23
Selected reaction monitoring is most selective. One ion from the first mass
separator is passed through a collision cell, where it breaks into several product
ions that are separated by a second mass separator. The intensities of one or a
few of these product ions arc plotted as a function of elution time. Thc selectivity
is high because few species from the column produce thc first selected ion and
even fewer break into the same fragments in the collision cell. This technique is
so selective that it can transform a poor chromatographic separation into a highly
specific determination of one component with virtually no interference.
23-8.
Column (a): hexane < butanol < benzene < 2-pentanone < heptane < octane
Column (b): hexane < heptane < butanol < benzene < 2-pentanone < octane
Column (c): hexane < heptane < octane < benzene < 2-pentanone < butanol
23-9.
Column (a): 3,1,2,4,5,6; Column (b): 3,4,1,2,5,6; Column (c): 3,4,5,6,2,1
23-10.
(a) (t = 8.4-3.7 = 4.7 min; k = 4.7/3.7 - 1.3
(b) * = KVJVm => K = (1.3X1.4) = 1.8
23-11
JU-1I.
23-12.
/ = 100 T8 + Í9 8^ lQfi( » 2.0)-lofi(l 1.0)1
1 IUUL8 + (9-S) log(14.0)-log(11.0)J •
836
log (15.0) = j f j + />
_
\ => a '- 1.69 x 10? K
log (20.0) = 3^3+ /j[
b -- -3.36
To solve for a, subtract one equation from the other to eliminate b. Once you
have a, substitute it back into either equation and solve for b.
1.692x 103
At 353 K: log/Y =
353
-3.36 => ft = 27.1 min
23-13.
Derivatization uses a chemical reaction to convert analyte into a form that is
more convenient to separate or easier to detect. In Box 23-1, amino and
carboxylatc groups of amino acids were converted to covalent derivatives to
make the molecules volatile enough to be separated by gas chromatography:
C02H
Amino
acid
^\wtn
O
C02CH,CH3
J<
Juin*
H
volatile
derivative
Gas Chromatography
23-14.
301
(a) In solid-phase microextraction, analyte is extracted from a liquid or gas into
a thin coating on a silica liber extended from a syringe. After extraction, the
fiber is withdrawn into thc syringe. To inject sample into a Chromatograph,
the metal needle is inserted through the septum and thc fiber is extended into
the injection port. Analyte slowly evaporates from the fiber in the hightemperature port. Cold trapping is required to condense analyte at the start
of the column during slow evaporation from the fiber. If cold trapping were
not used, thc peaks would be extremely broad because of the slow
evaporation from the fiber. During solid-phase microextraction, analyte
equilibrates between the unknown and the coating on the fiber. Only a
fraction of analyte is extracted into the fiber.
(b) In stir-bar sorptive extraction, a thick coating on thc outside ofa glasscoated stirring bar is used in place ofa thin coating on a fiber. After
extraction, thc bar is placed in a thermal desorption tube where analyte is
vaporized and cold trapped for chromatography. Thc volume of the coating
is -100 times greater in stir-bar sorptive extraction, so thc sensitivity is -100
times higher.
23-15.
Thc idea of purge and trap is to collect all of the analyte from thc unknown and
to inject all of the analyte into the chromatography column. Splitlcss injection is
required so analyte is not lost during injection. Any unknown loss of analyte
would lead to an error in quantitative analysis.
23-16.
The order of decisions is: (1) goal of the analysis, (2) sample preparation
method, (3) detector, (4) column, and (5) injection method.
23-17.
(a) A thin stationary phase permits rapid equilibration of analyte between the
mobile and stationary phases, which reduces the C term in thc van Deemter
equation. A thin stationary phase in a narrow-bore column gives small plate
height and high resolution. In a wide-bore column, the large diameter of the
column slows down thc rate of mass transfer between the mobile and
stationary phases (because it takes time for analyte to diffuse across the
diameter of the column), which defeats thc purpose of the thin stationary
phase.
302
Chapter 23
(b) Narrow-bore column: plate height = 1/(5 000 m-') = 2.0 x IO4 m »200 pm.
Thc area ofa length (£) of the inside wall of the column is itdi, where d is
thc column diameter. The volume of stationary phase in this length is tzdtt,
where / is the thickness of the stationary phase. For ¿ = 250 pm, t = 200
pm, and t = 0.10 pm, the volume is 1.57 x 104 pm3. A density of 1.0 g/mL
is 1.0g/cm 3 =1.0g/(10 4 pm) 3 =1.0g/10 12 pm 3 =l pg/pm3. The mass of
stationary phase in one theoretical plate is (I.57 x io 4 (¿m3)(l pg/pm3) =
I.57 x 104 pg. 1.0 % of this mass is = 0.16 ng.
Widc-borc column: For d = 530 pm, t = 667 pm, and / = 5.0 pm, the
volume is 5.5s x IO6 pm3. Mass of stationary phase is
(5.55 x 106 pm3)(l pg/pm3) = 5.55 x \ofi p g . 1.0% of this mass is = 56 ng.
23-18.
Use a narrower column or a longer column (doubling thc length increases
resolution by -^2) or try a different stationary phase.
23-19.
(a) The column on a chip is part ofa system intended to be an autonomous
environmental monitor. Therefore, it needs to be compact and to require
little power and consumables. Air is selected as carrier gas because it can be
taken from the atmosphere. Any other carrier gas wouldrequirea supply
tank which would be heavy, bulky, and would run out of gas. Oxygen from
air could degrade thc column at elevated temperature. Therefore, the
temperature must be kept below the point at which oxidation would occur.
Air has impurities which must beremovedby afiltrationsystem. The filter
is most likely a consumable which eventually needs replacement.
(b) The optimum velocity gives the lowest plate height. It is the minimum in
each curve. Optimum velocity = 9.3 cm/s for air and 17.6 cm/s for H2. Plate
height at optimum velocity = 0.036 cm for air and 0.051 for H2. (Values
comefromthc original publication. You will probably measure somewhat
different values from the figure.)
(c) Plates = column length/plate height = 3.0 m/0.036 cm = 8 300 for air and
5 900 for H2
(d) Time = column length/optimum velocity = 3.0 m/9.3 cm/s = 32 s for air and
17 s for H2
303
Gas Chromatography
(e) The two terms describe broadening due to thefinitetime for solute to diffuse
through thc stationary phase and the mobile phase. If the stationary phase is
sufficiently thin, the time for diffusion through the stationary phase (the Cs
term) becomes negligible.
(f) Acceptable flow rates for H2 are higher than for air because solutes diffuse
through H2 faster than they diffuse through air. With H2 carrier, solutes can
diffuse from thc center of the column to thc wall morerapidlythan they can
with air carrier.
23-20.
„
234 mg/ 88.15 g/mol
____ . .
(a) S = [pentanol] =
lO.OmL
= 0.2655 M
X = [2,3-dimethyl-2-butanol] = 2 3 ? "VolomU " " = 0 2 3 2 « M
Ax
JA£\
1.00
0.913 ^
r(
F = 1.253
[X] ~ F{[$V =* [0.232o M] "= t UO.2655 M]J
(b) I estimate the areas by measuring the height and wi/2 in millimeters. Your
answer will be different from mine if thefiguresize in your book is different
from that in my manuscript. However,relativepeak areas should be the
same,
pentanol: height = 40.1 mm; w\/2 • 3.7 mm;
area = 1,064 x peak height x W\a = 15g mm2
2,3-dimethyl-2-butanol:
height = 77.0 mm; w]/2 = 2.0mm; area = 164 mm2
(C)
2,3-dimetnyl-2-butanol
= ! 253
"
([93.7 mM])
=> [2,3-dimcthyl-2-butanol] = 77.6 mM
,,«
23-21.
395
( 787 ^ => F = 1.59
Ó2L Jâ$\
- t ^200 nM]j
[ x ] - E [^A) => [ 6 3 jM]
Thc concentration of internal standard mixed with unknown is
M&.6*10-5M)=0.16pM
633
[iodoacetone] = »-^ Qo."^Mj) => ^doacetone] -0.12 2 pM
[iodoacetone] in original unknown = "3 QQ (0.122 pM) = 0.41 pM
23 22
23-22.
/ - i n n 17? + n o 71 l o g ( 2 0 0 ) l o g ( 1 2 6 ) 1 = 932
yj¿
/ - 100 [(7 + (10-7) | o g (22.9) - log (12.6)J
304
23-23.
Chapter 23
(a) NaCI lowers thc solubility of moderately nonpolar compounds, such as
ethers, in water. Adding NaCI increases the fraction of the organic
compounds that will be transferred to the extraction fiber.
(b) Selected ion monitoring is measuring ion abundance for m/z 73. Only three
compounds in the extract have appreciable intensity at m/z 73.
(c) The base peak for both MTBE and TAME is at m/z 73. This mass
corresponds to M-15 (loss of CH3) for MTBE and M-29 (loss of C2H5) for
TAME. Loss of the ethyl group bound to carbon in TAME suggests that the
methyl group lost from MTBE is also bound to carbon, not to oxygen. If
methyl bound to oxygen were easily lostfromMTBE and TAME, we would
expect to see the ethyl group bound to oxygen lost from ETBE. There is no
significant peak at M-29 (m/z 73) in ETBE, The following structures are
suggested:
MTBE
ETBE
TAME
4.
—O-
•0-
^
—o-
—o-
mklZ
Base peak
m/z 57
m/z 87
f-
—o-
m/zTh
Base peak
m/z87
X
m/z 71
m/z 55 = C4H7*?
HO—
m/z 59
59 I
Base peak
23-24.
(a) The vial was heated to increase thc vapor pressure of the analyte and the
internal standard, so there would be enough in the gas phase (thc hcadspace)
to extract a significant quantity with thc microextraction fiber.
(b) At 60°C the analyte and internal standard are cold trapped at the beginning of
the column. Since desorption from thefibertakes many minutes, we do not
want chromatography to begin until desorption is complete.
305
Gas Chromatography
(c)
(d)
uO
N
I
CH 3
A
|
1 Least-Squares Spreadsheet
2
x
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
For 5-aminoquinoline,
m/z 144 is thc molecular
ion,C9H8NC5H,oN2+
C 5 HioN + , m/z 84
C
B
D
|
E
,
y
12
12
51
51
102
102
157
157
205
205
0.056
0.059
0.402
0.391
0.684
0.669
1.011
1.063
1.278
1.355
Highlight cells B16:C 18
Type "» LINEST(C4:C13,
B4:B13,TRUE.TRUE)
For PC. press
CTRL+SHIFT+ENTER
For Mac, press
I
COMMAND+RETURN LINEST output:
0.0222 b
m 0.006401
0.0234 sb
Sin 0.000185
"R1
00409 h
0.9933
n =
Mean y =
Z(xi-meanxf =
Measured y =
k = Number of replicate
measurements of y =
Derived x =
s,.=
10 B20 = CO JNT(B4:B1 3)
0.6968 B21 = AVI:RAGE(C4; C13)
48554.4 B22 = DEVSQ(B4:B13)
1.25 Input
2 Input
191.83 B26 = (B2 4-C16)/B16
5.54
I
„_
A
ft
28 B27 = (C18/B16)*SQRT((1/B25)+(1/B 20)+((B24-B21) 2V(B16 2'B22))
Least-squares parameters arc computed in the block B16:C18. In cell B24,
we insert the mean y value (1.25) for 2 replicate unknowns. The number of
replicates is entercd in cell B25. The derived value of x is computed in cell
B26 and the uncertainty is computed with Equation 4-27 in cell B27.
Answers for thc unknowns:
nonsmoker: 78 + 5 pg/L
nonsmoker with smoking parents: 192 ± 6 pg/I.
306
Chapter 23
x
1?
ni
OR
03
/
0
50
100
150
200
250
x
23-25.
Nitrite: ["NOj ] = [15N0¿ W - Äblank) = [80.0 pM](0.062 - 0.040) - 1.8 pM
Nitrate: ['«NO] ] = [^NO] }(R - Rbiank) = [800.0 pM](0.538 - 0.058) = 384 pM
23-26.
(a) The A term describing multiple flow paths is 0 for an open tubular column.
Multiple paths arise in a packed column when liquid takes different paths
through thc column.
(b) B = 2Dm, where Dm is the diffusion coefficient of solute in the mobile phase.
(c) C = Cs + Qm
2k d2
1 +6k+Uk2 r2
C
~ Xk+lpDs
n« - 24(A+1)2 D m
where k = retention factor
d = thickness of stationary phase
r = column radius
Ds = diffusion coefficient of solute in the stationary phase
Dm = diffusion coefficient of solute in the mobile phase
(d) H = B/ux + Cux
(ux • linear velocity)
r
Cs
Plate height is a minimum at thc optimum velocity:
dH_
_B_
dux * - ux
2 + C =
[B
u
° =* * (°P
timum
)
=
'\]c
The minimum plate height is found by plugging this value of ux (optimum)
back into the van Deemter equation:
//min = B/ux +Cux = B * y f + C y § = 2^BC = 2^B(C% + Cm)
307
Gas Chromatography
f 2k
cfl 1 +6A+ 1U 2 r^
//min - 2\J (2/>m)^3(A + ])2 Ds+ 24(k + 1 ) 2 An.
j
//min
=s
I 4/V ^¿»m ( 1 + 6¿ + 1 lit2) 2/^
2 ^ / 3 ( ^ + 1 ) 2 Ds +
24(* + I) 2
23-27. (a) As*-> 0, * W = VÑ3 » 0.58
/1 + 6A+1U2
As*->»,JWi' = '\J 3(i+jfc)2
fñ&
-+^3#
. /IT
V3
1Q
l9
(b) As it -» 0, /7min = 0-58 r = 0.058 mm
As it-* oo, Hm\n = 1.9 r = 0.19 mm
/l + 6-5.0+ IK25
3^35)
= 1-68 r = 0.168 mm
(c) For it = 5.0,/7 mm = r AJ
50 x 103 mm
_A
Number of plates - 0 .168 mm/platc " 3 0 X
1AS
10
(d) k = A:Ks/Km, where Vs is the volume of stationary phase and Vm is thc
volume of mobile phase. For a length of column, I, the volume of mobile
phase is %r2i and the volume of stationary phase is 2%rti. Substituting these
volumes into the equation for k gives k = KQnrtíy^t)
_ 2 (0.20 urn) (1000)
k
(100 pm)
23-28.
- 2tK/r.
The van Deemter equation has thc form
H = BluK + Cux = BIux + (C s + Cm)wx
2k
d2
1 + 6k+Uk2
r2
Cs
2
Cm
2
B = 2Dm
~'~ 3(k+\) E>s
~~
24(k+l)
Dm
where
k = retention factor = 8 . 0
d= thickness of stationary phase = 3.0 * 10 -6 m
r = column radius = 2.65 x lO^m
D s = diffusion coefficient of solute in the stationary phase
Dm = diffusion coefficient of solute in thc mobile phase
Experimentally, we find // = (6.0 x 10-5 m2/syUx + (2.09 x l()-3 s)wx.
Therefore, B = 2Dm = (6.0 x 10-5 m 2/ s ) f o r om = 3.0 x 10-5 m 2/ s .
From thc second term of thc experimental van Deemter equation, we know that
2k
d2
1 + 6 / c + I U 2 r2
+
Cs + Cm = 2.09x10-3 s = 3 ^ - ^ 2 Ds
24(k+\)2
Pm
Inserting thc known values of all parameters allows us to solve for D s :
2.09 x 10-3 s =
308
Chapter 23
2(8.0)
(3.0 x lQ-6 m ) 2
3((8.0)+ 1)2
£>s
i+
j 1( g 0 ) 2 (2.65 x 1Q-4 m)2
24((8.0)+l) 2 ' (3.0 x 10-5 m 2/ s )
6 ( 8 Q) +
=> Ds = 5.0 x 10- |0 m 2 /s
The diffusion coefficient in the mobile phase is (3.0 x 10-5 m2/s)/(5.0 x 10-10
m2/s) = 6.0 x in4 times greater than thc diffusion coefficient in the stationary
phase. This makes sense, because it is easier for solute to diffuse through He gas
than through a viscous liquid phase.
23-29.
(a)
K = 1 0 000
K = 5 000
8
300
K = 1 000
I
20
40
K = 100
i
60
l.
80
100
Solution volume (mL)
Mass of analyte extracted by solid phase microextraction
V.(mL)
K = part it ion
m (ng)
coefficient =
0
1.00E+02
V, = volume ot
1
0.000
6.455
2
6.670
3
4
6.745
6.783
5
6.806
10
50
100
6.853
6.890
film (mL) =
6.90E-04
Co = initial
concentration
in solution
(ug/mL) =
6.895
0.1
1000
6.900
C4 = 1000*($A$5*$A$8*$A$13*B4)/($A$5»$A$ 3 + B4)
(b)
m
=
KVfC0Vs
KVf+ Vs
If V
*»
KV m
t
=
Kv c
fo
309
Gas Chromatography
For Vf = 6.9 x IO-4 mL and c0 = 0.1 pg/mL, m - • (6.9 x 10-5)(/Q pg
For K = 100, m —• 6.9 ng, which agrees with thc graph.
For K = 10000, m —* 690 ng, which is where the graph is heading, but it will
require about 1 liter of solution to attain the limiting concentration in the
fiber.
(c) Thc spreadsheet tells us lhat when K = 100, 6.85 ng have been extracted into
thc fiber and when A" = 10 000,408 ng have been extracted into thc fiber.
Thc total analyte in 10.0 mL is (0.10 pg/mL)(10.0 mL) = 1.0 pg. The
fraction extracted for K = 100 is 6.86 ng/1.0 pg = 0.006 9 (or 0.69%). The
fraction extracted for K = 10 000 is 0.41 (or 41 %).
23-30.
(a) For the formula C9H4N2CI6,
rings + double bonds = c-h/2
+ M/2 + 1 = 9 - (4+6)/2 + 2/2 + 1 = 6,
which agrees with the structure that has 2 rings + 4 double bonds.
(b) Nominal mass = integer mass of thc species with thc most abundant isotope
of each of the constituent atoms. For C9H4N2G6, nominal mass = (9 * 12)
+ ( 4 x l ) + (2x 14)+ (6x35) = 350.
(c) Thc sequence m/z 350, 315, 280, 245, and 210 corresponds to successive
losses of mass 35 Da. A logical assignment is C9H4N2G6, C9H4N2CI5,
C9H4N2CIÎ, C9H4N2CI5, C9II4N2CIJ.
Chapter 23
(d) Here is the spreadsheet for 5 Cl atoms:
A
B
C
D
1 Isotopic abundance from binomial distribution
2
3;
'CI =
3
0.7577 natural abundance
E
4
5
6
17
7
*CI
8
5
Vcio
M
0.24974
62.54
9
4
»Cl/'CI,
M+2
0.39931
100.00
10
3
"cV'ci,
M+4
0.25539
63.96
11
2
^CI^CI,
M+6
0.08167
20.45
ci, cu
M+8
0.01306
3.27
^Clo^CIs
M+10
0.00084
0.21
CI =
n=
0.2423 natural abundance
5
Relative
12
1
13
0
Formula
35
37
Mass
Abundance abundance
14 D8 = BINOMDIST(A8,$B$5,$B$3,FALSE)
15 E8 = 100*C 8/MAX($D3>8:$D$13)
And here are the results for species with 6, 5,4, 3, 2, and 1 Cl atom. The
predicted patterns are in reasonable agreement with thc observed amplitudes
of thc clusters of peaks at m/z 350, 315, 280, 245, and 210.
M
M+2
Cl 6
52.12
M+4
100.00
79.95
M+6
M+8
34.09
8.18
M+10
1.05
0.06
M+12
Predicted relative abundance
CIs
CU
Cl 3
Cb
62.54
78.18
100.00
100.00
100.00
100.00
95.94I
63.96
63.96
47.97
30.68
10.23
20.45
10.23
3.27
3.27
0.21
0.82
Cl,
100.00
31.96
CHAPTER 24
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY
24-1.
(a) In reversed-phase chromatography, the solutes are nonpolar and more
soluble in a nonpolar mobile phase. In normal-phase chromatography, the
solutes are polar and more soluble in a polar mobile phase.
(b) A gradient of increasing pressure gives increasing solvent density, which
gives increasing eluent strength in supercritical fluid chromatography.
24-2.
Solvent is competing with solute for adsorption sites. The strength of the
solvent-adsorbent interaction is independent of solute.
24-3.
In hydrophilic interaction chromatography, solute equilibrates between the
mobile phase and an aqueous layer on thc surface of the polar stationary phase.
The more water in the eluent, the better can eluent compete with the stationary
aqueous layer to dissolve polar solute and elute it from the column.
24-4.
(a) Small particles give increasedresistanceto flow. High pressure is required
to obtain a usable flow rate.
(b) A bonded stationary phase is covalently attached to the support.
24-5.
(a) ¿(cm) «
Ndj^iim)
3 nno
If N = 1.0 x io 4 and dp = 10.0 pm, L = 33 cm
dp = 5.0pm => L= 17cm;
dp= 3.0pm => L= 10cm
dp= 1.5 pm => ¿ = 5cm
(b) Efficiency increases because solute equilibrates between phases more
rapidly if the thicknesses of both phases are smaller. This effect decreases
the C term in the van Deemter equation. Also, migration paths between
small particles are more uniform, decreasing the multiple path (A) term.
24-6.
Plates (AO = (15 cm)/(5.0 x in-4 cm/plate) = 3.0 x IO4
555
ñ
/5T55
/ 5.55
N - - ^ 2 ~ => wi/2 = hyj-ff-
- ( 1 0 . 0 m i n ) > J 3 0 x l 0 4 = 0.136min
If plate height = 25 pm, plates = 6 000 and w 1/2 = OJO4 min
311
312
24-7.
Chapter 24
Silica dissolves above pH 8 and thc siloxane bond to thc stationary phase
hydrolyzes below pH 2. Bulky isobutyl groups hinder thc approach of H30 + to
the Si-O-Si bond, so the rate of acid-catalyzed hydrolysis is decreased.
24-8.
The high concentration of additive binds to the sites on the stationary phase that
would otherwise hold on tightly to solutes and cause tailing.
24-9.
(a) Your sketch should look like Figure 22-14, in which the asymmetry factor is
AIB = 1.8, measured at one tenth of the peak height.
(b) Tailing of amines might be eliminated by adding 30 mM triethylamine to the
mobile phase. Tailing of acidic compounds might be eliminated by adding
30 mM ammonium acetate. For unknown mixtures, 30 mM
tricthylammonium acetate is useful. If tailing persists, 10 mM
dimcthyloctylamine or dimethyloctylammonium acetate might be effective.
Tailing could also be caused by a clogged frit which you might be able to
clean by washing with reversed flow.
24-10. (a)
t
£
o>
1a>
ts
O-
H - A + B/Uv + Cu.
»1
il
i\
No B term
* \""
\ *\
\
No C term
Linear flow rate ->
(b) For 1.8-pm particle size, the experimental van Deemter curve looks almost
like the curve with no C term in (a) (that is, finite equilibration time ~ 0).
When particle size is small enough, equilibration between the mobile and
stationary phases is very rapid and this process contributes little to peak
broadening. The experimental curve for 1.8-pm particles levels off at a
smaller plate height than the curves for 5- and 3.5-pm particles. This
behavior suggests that the A term (multiple flow paths) is smaller for the
smaller particles.
313
High-Performancc Liquid Chromatography
(c) A superficially porous particle has a thin porous shell on a solid inner core.
Solute only needs to diffuse short distances into thc thin shell, so
equilibration occurs on a time scale similar to that of smaller particles.
However, the overall diameter of thc superficially porous particle is not
small, so its resistance to fluid flow is not as high as that ofa small particle.
555
24-11.
ñ
5.55 (4.70 min)2
, c£rt r
=
(a) N =
2~~ =
\
,
i
u
'
560 for / cnantiomer
m gH
n
v
4
'
w,^
(028 mm)5.55(5.37 min)2
.-.**
,
N = —TTTT—. V¿ = 1 310fori/cnantiomer
(0.35 min)
(b) W1/28V = '/2Í0.28 min + 0.35 min) = 0.315 min
0.589A/r
0.589 (5.37 min-4.70 min)
Resolution = „ ^
= "
0.315 min
'
125
(c) Unadjusted relative retention: y = (5.37 min)/(4.70 min) = 1.143
Average N = lA(\ 560+ 1 310)= 1435
Resolution - ^ (
24-12.
Y
- 1) = 2 ^ p ( 1 . 1 4 3 - 1) = 135
(a) P « —j
For two difference conditions (1 and 2),
P
P2
d2
( 3 mn \2
-5- oc —î = r-f 1 — = 18. Pressure must be 18 times greater.
Pi
d2
v.0.7 pm)
(b) « x oc /», so if pressure is increased by a factor of 10, then linear velocity
should increase by a factor of 10.
(c) Mass transfer between the mobile and stationary phase is faster for small
particles than for large particles. Thc optimum velocity for maximum
efficiency (highest plate number) increases as the rate of mass transfer
increases. In the example cited, thc high flow rate is closer to the optimum
flow rate than is the low flow rate.
24-13.
(a) Bonded reversed-phase chromatography
(b) Bonded normal-phase chromatography (Dioxane is closer to ethyl acetate
than to chloroform in eluent strength.)
(c) Ion-exchange or ion chromatography
(d) Molecular-exclusion chromatography
314
Chapter 24
(c) Ion-exchange chromatography
(f) Molecular-exclusion chromatography
4
4
24-14.
IO-pm-diameter spheres: volume = 3 TTT = T JI(5 X i o 4 cm)3 = 5.24 x IO"10cm3
Mass of one sphere = (5.24 x IO 1 0 mL)(2.2 g/mL) = 1.15 x 10-9 g
Number of particles in l g = l g / ( 1 . 1 5 x 10"9 g/particle) = 8.68 x io 8
Surface area of one particle = 4îtr2 = 4n(5 x IO 6 m)2 = 3.14 x 10-10 m 2
Surface area of 8.68 x 108 particles = 0.27 m2
Since the observed surface area is 300 m2, the particles must have highly
irregular shapes or be porous.
24-15.
(a) Since the nonpolar compounds should become more soluble in the mobile
phase, the retention time will be shorter in 90% methanol.
3
(b) At pH 3, the predominant forms are neutral RCO2II and cationic RNHt.
The amine will be eluted first, since RNH* is insoluble in the nonpolar
stationary phase.
24-16.
(a) Unretained component travels at the solvent velocity, ux.
_ column length
4400 mm
transit time ~ (41.7 min)(60 s/min)
Wx =
fM
\°)
t
K
- t'-t™ - 188-1 min -41.7 min
t
~
.41.7
1 1 7 m,'„
min
lJ6
->•->'
5 55/
rt22
' ?
5.55(188.1 min)
~^h =
0 01 miny
» 192 000
„
4 400 mm
H
" 192000 - 2 2 9 ^ m
/ ^ x,
(C)
-
=
MI D 1 ,
°- S89A 'r
0.589(1.01 min)
A rM
(d) Resolution = - ^
=
1.01 m i n
- 0.589
,
s
(C)
a
=
'¿
Z
'r2
y =^
=
=
194.3 min-41.7 min
193.3 min-41.7 min =
194.3 min
1933Ä - 1 0 0 5 *
1006
*
mm/s
315
High-Performance Liquid Chromatography
(0
Resolution = 4 ( y - 1 )
1.000 = ^ p (1.0052-1) => N = 5.9 2 x 10s
A column length of 440 cm gave N - 1.92 x 10s plates. To obtain 5.92 x IO5
plates, the column must be longer by a factor of T ^ h ^ p t o t e l
=
3
°8,
Required length = (3.08)(4.40 m) = 13.6 m
(g) Slow the flow rate to possibly decrease H and thereby increase N.
Change the solvent to change the relative retention.
(h) Resolution = ^ ( y - 1) = ^ p ^ ( 1 . 0 0 8 3 - 1) = 0.91
24-17.
(a) On (/?,/?)-stationary phase, (5)-gimatecan is elutcd at 6.10 min. On
(5,5)-stationary phase, (5)-gimatecan is retained more strongly and is elutcd
at 6.96 min. (Ä)-gimatecan must have the exact opposite behavior. It will be
eluted at 6.96 min from (/?.tf)-stationary phase and at 6.10 min from
(S,5)-stationary phase.
(b) With (S,S)-stationary phase, we observe a small peak at 6.10 min for
(tf)-gimatccan. This peak is well separated from the front of the big
(5>gimatccan peak centered at 6.96 min, so the two areas can be integrated
and compared with each other. With (Ä.Ä)-stationary phase, wc see the
(5)-gimatecan peak at 6.10 min with no evidence of the minor (Ä)-gimatecan
peak at 6.96 min. Thc minor peak is lost beneath the tail of (5)-gimatecan.
Chromatography on each enatiomer of thc stationary phase enables us to
unambiguously locate where each enantiomer of gimatecan is elutcd, even
though we do not have a standard sample of (Ä)-gimatecan.
(c) For the (S,.S>stationary phase, we have thc following information:
(S)-gimatecan: tT = 6.96 min
(Ä)-gimatecan: tT = 6.10 min
k = 1.50
k = 1.22
The definition of retention factor is k - (tT - / m )// m - Inserting A: = 1.50 and
t, = 6.96 min for (5)-gimatecan gives tm = 2.784 min. We should get the
same value of/m for (>()-gimatecan by inserting k = 1.22 and U - 6.10 min
into the equation k = (tT - tmytm- In fact, this pair of numbers gives /m 2.74s min. The difference is from experimental error plus roundoff. Let's
take the average value /m • 2.766 min.
316
Chapter 24
The adjusted retention time is tt - tr - tm.
(S)-gimatecan: tt = tr-tm
= 6.96-2.76 8
4.l9 2 min
(Ä)-gimatccan: tT = tT-tm
= 6.10-2.76« = 3.332min
n i *•
. .•
'r2
4.19 min
Relative retention: a = — = •, •»•»'2 ¡H = 1.25«
t ,
J.JJ2 rain
Unadjusted
relative retention: y' = —
J
/ r l - T^TTi—~
6.10 mm = 1.14i'
(d) Resolution = 4 ( y - 1) =
4—(1.14, - 1) = 2.91 which is more than
adequate for "baseline" separation. Tailing of thc peaks creates a little
overlap, but it should not be very serious for an equal mixture of the
cnantiomcrs.
24-18.
Peak areas will be proportional to molar absorptivity, since thc number of moles
of A and B are equal.
Area of A
2.26 x lp4
Area of B " 1.68 x io 4
=
1.064 xA A w 1/2
1.064 x Äßwi/2
=
(128)(I0.I)
/?B (7.6)
=> Aß — 126 mm
24-19.
Acetophcnonc is neutral at all pH values. Its retention is nearly unaffected by
pH. For salicylic acid, we expect thc neutral molecule, HA, to have some affinity
for the Cs nonpolar stationary phase and thc ion, A*, to have little affinity for C«.
Salicylic acid is predominantly HA below pH 2.97 and A" above pH 2.97. At pH
3, there is nearly a 1:1 mixture of HA and A-, which is moderately retained on the
nonpolar column. At pH 5 and 7, more than 99% of the molecules arc A% so
retention is weak (small retention factor).
OH
A"
Q
lili
Ionic forms of nicotine ought to have low affinity for the nonpolar stationary
phase and the neutral molecule would have some affinity. Abbreviating nicotine
as B, the form B is dominant above pH - pA'2 = 7.85. BH+ is dominant between
pH 3.15 and 7.85. BH2,* is dominant below pH 3.15. B does not become
317
High-Performance Liquid Chromatography
appreciable until pH - 7, so the retention factor is low below pH 7 and increases
at pH 7.
24-20.
(a) Vm « 0.5 L d2 = 0.5(5.0 cm)(0.46 cm)2 = 0.53 cm 3 = 0.53 mL
'm " VnJF • (0.53 mL)/( 1.4 mL/min) = 0.38 min for column A
= (0.53 mL)/(2.0 mL/min) = 0.26 min for column B
(b) Morphine 3-ß-D-glucuronide is more polar than morphine because of the
added hydroxyl groups and the carboxylic acid. The more polar compound
is less retained by the nonpolar reversed-phase column.
(c) Bare silica is a polar, hydrophilic surface. Morphine should not be retained
as strongly as the more polar morphine 3-ß-D-glucuronide. The gradient
goes to increasing H2O for increasing polarity (that is, increasing solvent
strength) to remove the more strongly adsorbed, more polar compound.
(d) k =
r
m
=
'
Q65—
,
tT-tm
2.8-0.65
= —ñ«—
k = ~Z—
rm
0.65
= 1.3 for morphine 3-ß-D-glucuronide
=
3.3 for morphine
(c) Km = Ftm = (2.0 mL/min)(0.50 min) = 1.0 mL
içE
_ (5.0 min)(2.0 mL/min)
k
=
~ A4>VmS
(0.4)(1.0mL)(4)
24-21.
(a) Electrical power - current x voltage. Current is the rate of flow of charge
through a circuit. It is analogous to the rate of flow of liquid through a
column. Voltage is the potential difference driving charge through the wire.
It is analogous to the pressure difference driving liquid through a column.
(b) 1 mL = 1 cm3 = (10 2 m)3 = IO* m3
1 mL/min = IO-* m3/60 s = 1.67 x 10 s m3/s.
3 500 bar = 3 500 x 10s Pa - 3.5 x 108 Pa
power = volume flow rate x pressure drop
= (1.67 x 10'* m3/s)(3.5 x 10* Pa) = 5.8 W
318
24-22.
Chapter 24
(a)
Hg
H
3
J\\/C0 2 CH 3
CocainctT
C I 7 H 2 2 N0 4
m/z 304
Y
C6H5
()
(b) The C6H5CO2 group has a mass of 121 Da. Subtracting 121from304 gives
183 Da. The peak at m/z 182 probably represents cocaine minus
C6H5CO2H. The structure might be thc one below or somerearrangedform
of it.
C,oH| 6 N02
« 4 182
r
H
(c) The ion at m/z 304 was selected by massfilterQl. Its isotopic partner
containing l3C at m/z 305 was blocked by Ql. Because the species at m/z
304 is isotopically pure, there is no 13C-containing partner for the
collisionally activated dissociation product at m/z 182.
(d) For selected reaction monitoring, the massfilterQl selects just m/z 304,
which eliminates components of plasma that do not give a signal at m/z 304.
Then this ¡on is passed to the collision cell, in which it breaks into a major
fragment at m/z 182 which passes through Q3. Few other components in thc
plasma that give a signal at m/z 304 also break into a fragment at m/z 282.
Thc 2-step selection process essentially eliminates everything else in the
sample and produces just one clean peak in the chromatogram.
319
High-Pcrformance Liquid Chromatography
(e) The phenyl group must be labeled with deuterium because the labeled
product gives the same fragment at m/z 182 as unlabeled cocaine.
CH3
N
JV.CO2CH3
CO2CH3
Q
C 17 D 5 H l7 N0 4
m/z 309
CioHieNC^
m/z 182
11
(f) First, we need to construct a calibration curve to get the response factor for
cocaine compared to 2H5-cocaine. We expect this response factor to be
close to 1.00. We would prepare a series of solutions with known
concentration ratios [cocaine]/[2Hs-cocaine] and measure the area of each
chromatographic peak in the chromatography/atmospheric chemical
ionization/selected reaction monitoring experiment. A graph would be
constructed, in which [peak area of cocainc]/[pcak area of 2Hs-cocainc] is
plotted versus [cocainc]/[2H5-cocaine]. Thc slope of this line is thc response
factor.
03
a
•
•
a.
8
Slope =
response factor
c
[cocaine]
[2H5-cocaine]
For quantitative analysis, a known amount of thc internal standard 2H5cocaine is injected into thc plasma. From the calibration curve, the relative
peak areas tell us the relative concentrations of cocaine and the internal
standard. From the known quantity of internal standard injected into thc
plasma, we can calculate the quantity of cocaine.
320
24-23.
Chapter 24
(a) Atmospheric pressure chemical ionization gives a prominent peak at m/z
234, which must be MH + . The peak at m/z 84 is probably the fragment
C5HJON + , which might have thc structure shown below.
Q
MH+
/w/z234
Str^
c H,oN+
5
m/zU
H
In selected reaction monitoring, m/z 234 is selected by mass filter Ql and
m/z 84 is selected by mass filter Q3 in a triple quadrupolc spectrometer.
(b) Deuterated internal standard has the formula C H H | 6 2 H 3 0 2 N , with a
nominal mass of 236. The protonated molecule is m/z 237. Cleavage of the
C-C bond gives the same C5H10N4 fragment as unlabeled Ritalin. The
transition to monitor is m/z 237 ->• 84.
Q
C0 2 CD 3
m/z 237
24-24.
m/z 84
(a) To find k, measure the retention time for the peak of interest (/r) and the
elution time for an unretained solute (/m). Then use thc formula
k = (tT - tm)ftm. The resolution between neighboring peaks is the difference
in their retention lime divided by their average width at thc baseline.
0>) (') ka is usually the time when the first baseline disturbance is observed,
(ii) Unretained solutes such as uracil or sodium nitrate could be run and
observed with an ultraviolet detector, (iii) Alternatively, the formula
/ m ~ Ld2 l(2F) can be used, where /, is the length of thc column (cm),
dc is the column diameter (cm), and F is the flow rate (mL/min).
(c) tm*Ldll(2F)
= (15X0.46)2/(2-1.5) = l.0 6 min
tm docs not depend on particle size. The estimate is 1.0^ min for both 5.0and 3.5-pm particles.
24-25.
Dead volume is the volume of the system (not including the chromatography
column) from the point of injection to the point of detection. Dwell volume is the
volume of the system from the point of mixing solvents to the beginning of the
High-Performance Liquid Chromatography
321
column. Excessive dead volume causes peak broadening by longitudinal
diffusion. In gradient elution, dwell volume determines the time from the
initiation of a gradient until the gradient reaches the column. The greater the
dwell volume, the more the delay between initiating a gradient and the actual
increase of solvent strength on the column.
24-26.
A rugged procedure should not be seriously affected by gradual deterioration of
the column, small variations in solvent composition, pH, and temperature, or use
ofa different batch of thc same stationary phase. A procedure should be rugged
so that inevitable, small variations in conditions do not substantially affect the
outcome of the separation.
24-27.
0.5 <k<20; resolution > 2; operating pressure < 15 MPa;
0.9 < asymmetry factor < 1.5
24-28.
Run a wide gradient (such as 5%B to 100%B) in a gradient time, /G selected to
produce k* « 5 in Equation 24-10. Measure the difference in retention time (At)
between the first and last peaks eluted. Use a gradient if AtltQ > 0.25 and use
isocratic elution if AtltQ < 025.
24-29.
The first steps are to ( 1 ) determine the goal of thc analysis, (2) select a method of
sample preparation, and (3) choose a detector that allows you to observe the
desired analytes in the mixture. The next step could be a wide gradient elution to
determine whether or not an isocratic or gradient separation is more appropriate.
If the isocratic separation is chosen, %B is varied until criteria for a good
separation are met. If adequate resolution is not attained, you can try different
organic solvents. If adequate resolution is still not attained, you can use a slower
flow rate, a longer column, smaller particles, or a different stationary phase.
24-30.
To use two organic solvents (A and B), the optimum concentration of A is first
found to get the best separation while keeping all retention factors in thc range
0.5-20. If adequate separation docs not result, then thc same procedure is carried
out with solvent B. If adequate separation is still not attained, a 1:1 mixture of
the best compositions of A and B should be tried. If it looks promising, other
mixtures of the optimum concentrations of A and B can be tried.
24-31.
Chromatography is conducted with four conditions: (A) high %B, low T, (B)
high %B, high T, (C) low %B, high T, and (D) low %B, low T, Based on thc
322
Chapter 24
appearance of thc chromatograms, combinations between the points A, B, C, and
D can be explored for further improvement in the separation.
24-32.
Peak 5 has a retention time (/r) of 11.0 min for 50% B. The retention factor is k =
Cr - 'mV'm = (11.0- 2.7)/2.7 = 3.1. When B is reduced to 40%, the rule of three
predicts k = 3(3.1) = 9.3. Rearranging the definition of retention factor, we find tx
• 'm* + 'm = 'm(* + 1 )• We predict for 40% B / r = tm(k +]) = (2.7)(9.3 + 1 ) =
27.8 min. The observed retention time at 40% B is 20.2 min.
24-33.
(a)
%B
Retention time (min)
_peak 6
4.4
4.4
4.9
4.5
4.5
5.1
5.6
5.6
7.3
8.2
8.2
12.2
13.1
13.6
24.5
24.8
27.5
65.1
37.6
44.2
125.2
90
80
70
60
50
40
35
£
£
50
90
80
70
60
%B
50
40
30
At 45% B, we could estimate that Peak 8 will be eluted halfway between the
times for 40% B and 50% B, which is about 45 min. Thc fit to the curve above
suggests that 36 min is a more realistic estimate.
323
High-Performance Liquid Chromatography
(b) The table shows the calculation of retention factor k for Peaks 6-8.
<D
0.9
0.8
0.7
0.6
05
0.4
0.35
rm =
2.7 min
retention factor k = (f r -1 m)ftm
retention time t, (min)
Peak 6
Peak 7
Peak 8
Peak 6 Peak 7 Peak 8
0.815
0.630
0.630
4.4
4.4
4.9
0.667
0.889
0.667
4.5
4.5
5.1
1.074
1.704
1.074
5.6
5.6
7.3
3.519
2.037
2.037
8.2
8.2
12.2
4.037
8.074
3.852
13.1
13.6
24.5
23.111
9.185
8.185
24.8
27.5
65.1
12.926
15.370
45.370
37.6
44.2
125.2
og*
Peak 6
-0.201
-0.176
0.031
0.309
0.586
0.913
Peak 7
-0.201
-0.176
0.031
0.309
0.606
0.963
Peak 8
-0.089
-0.051
0.231
0.546
0.907
1.364
1.111
1.187
1.657
2.00
A
1.50
y= -4.4064X+ 3.1565
/ fit to 4 points at left
\
e \
1.00
0.50
3\ sA
(J
KCl) KO
a
Peak 7
A Peak 8
0.00
-0.50
—
0.3
i
—
0.4
i
—
0.5
i
—
0.6
— i — i —
0.7
0.8
i
0.9
—
1
Solvent composition (<I>)
The first obvious point is that log k versus d> does not follow a straight line
over a wide range of solvent composition. The straight line going through
the four points for Peak 8 from «P = 0.35 to 0.6 is log k = -4.4064d> +
3.1565. At O = 0.45, we compute log k= 1.1736 and k =14.92. We
compute /r = tm(k+\) = 43.0 min. If we had only taken the first three points
(<J> = 0.35 to 0.5), we would find log k = - 4 . 9 3 6 4 * + 3.3660. At <D - 0.45,
we compute log k = 1.11447, k - 13.95, and tT = 40.4 min.
Chapter
(a)
Solvent
composition
0.0
B
0.5
F
1.0
C
1
8.0
6.0
5.5
Retention times (min)
3
4
11.5
13.8
5.0
20.5
9.6
21.7
4.3
2
8.0
9.6
Predicted positions (by linear interpolation):
0.25
8.80
8.25
17.15
7.0
0.75
5.75
9.6
4.65
21.10
for Peaks
5
12.8
17.3
23.6
1-7
6
12.8
22.0
16.5
37.0
16.0
13.6
15.05
20.45
17.40
19.75
26.50
14.80
Peak I
IVA :
Peak 3
Peak 4
IVA n
Peak 6
Peak?
0.00
B
0.25
0.50
F
0.75
1.00
c
Position(BFC)
Midway
between
Band F
r
5
7
i^
10
15
20
Time (min)
25
-1
30
325
High-Performance Liquid Chromatography
Midway
between
FandC
5
i
r
10
15
Retention time
r
20
25
(b) B: 40% methanol/60% buffer
C: 32% tetrahydrofuran/68% buffer
F: 20% methanol/16% tetrahydrofuran/63% buffer
Between B and F: 30% methanol/8% tctrahydrofuran/62% buffer
Between F and C: 10% methanol/24% tetrahydrofuran/66% buffer
24-35.
D:
E:
F:
G:
25% acetonitrile/30% methanol/45% buffer
25% acctonitrile/20% tetrahydrofuran/55% buffer
30% methanol/20% tetrahydrofuran/50% buffer
16.7% acetonitrile/20% methanol/13.3% tctrahydrofuran/50% buffer
24-36.
In the nomograph in Figure 24-26, a vertical line at 48% methanol intersects thc
acetonitrile line at 38%.
24-37.
(a) Lower solvent strength usually increases the difference in retention between
different compounds. Use a lower percentage of acetonitrile.
(b) In normal-phase chromatography, solvent strength increases as thc solvent
becomes more polar, which means increasing thc methyl /-butyl ether
concentration. We need a higher concentration of hexane to lower the
solvent strength, increase the retention times, and probably improve
resolution.
24-38.
(a) AtltG - 19/60 = 0.32. Because A///G > 0-25, gradient elution is suggested.
(b) At / = 22 min, the solvent composition entering the column can be calculated
by linear interpolation: 5 + §§ (100 - 5) = 39.8%. At 41 minutes, the
composition is 5 +1¿ (100 - 5) = 69.9%». A reasonable gradient for thc
second experiment is from 40 to 70% acetonitrile in 60 min.
326
24-39.
Chapter 24
(a) (1) Change thc solvent strength by varying thc fraction of each solvent. (2)
Change thc temperature. (3) Change the pH (in small steps). (4) Use a
different solvent. (5) Use a different kind of stationary phase.
(b) Use a slower flow rate, a different temperature, a longer column, or a smaller
particle size.
24-40.
(a) Start with conditions to give k* = 5 and assume that S = 4 for molecules in
the mixture. Vm » Ld2/2 = (15 cm)(0.46cm)2/2 = 1.5omL. Particle
size does not come into the calculation.
G
w
_ k*A<S>VmS _ (5X0.9X1.59 mL)(4)
"
F
(1.0 mL/min)
^n
= 29 m m
tGF
_ (11.5 minKI.O mL/min)
A<!>VmS~
(0.14)(1.59mL)(4) ~
u
*
The large column has thc same length as the small column, but thc diameter
is increased from 0.46 to 1.0 cm. The volume increases by a factor of
(1.0/0.46)2 = 4.7. Therefore, wc increase the flow rate and the sample
loading by a factor of 4.7. Flow rate - 4.7 mL/min and sample load = 4.7
mg. The gradient time is unchanged at 11.5 min. For the large column, Vm
* Ld\l2 = (15cm)(1.0cm) 2 /2 = 7.5 mL and
tGF
_ (11.5 min)(4.7 mL/min)
Ad>VmS~
(0.14)(7.5mL)(4)
u y
CHAPTER 25
CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS
25-1.
The separator column separates ions by ion exchange, while the suppressor
exchanges the countcrion to reduce the conductivity of eluent. After separating
cations in the cation-exchange column, thc suppressor must exchange the anion for
OH", which makes H2O from thc HCl eluent.
25-2.
Increased cross-linking gives decreased swelling, increased exchange capacity and
selectivity, but longer equilibration time.
25-3.
Deionized water has been passed through ion-exchangers to convert cations to H +
and anions to OH", making H2O. Nonionic impurities (e. g., organic compounds)
are not removed by this process, but can be removed by activated carbon.
25-4.
One way is to wash extensively with NaOH a column containing a weighed amount
of resin to load all ion-exchange sites with OH". After a thorough washing with
water to remove excess NaOH, the column can be cluted with a large quantity of
aqueous NaCI to displace OH". Eluate is then titrated with standard HCl to
determine the moles of displaced OH".
25-5.
(a) As pH is lowered the protein becomes protonated, so the magnitude of the
negative charge decreases. The protein becomes less strongly retained.
(b) As the ionic strength of eluent is increased, the protein will be displaced from
thc gel by solute ions.
25-6.
Particles pass through 200 mesh (75 pm) sieve and are retained by 400 mesh
(38 pm) sieve. 200/400 mesh particles are smaller than 100/200 mesh particles.
25-7.
The pKa values are: NH^ (9.24), CH3NH+ (10.64), (CH3)2NH+ (10.77), and
(CH3)3NH+ (9.80). If the four ammonium ions arc adsorbed on a cation exchange
resin at, say, pH 7, they might be separated by elution with a gradient of increasing
pH. The anticipated order of elution is NH 3 < (CH3)3N < CH3NH2 < (CH3)2NH.
We should not be surprised if thc elution order were different, since stcric and
hydrogen bonding effects could be significant determinants of the selectivity
coefficients. It is also possible that elution with a constant pi I (of, say, 8) might
separate all four species from each other.
327
328
25-8.
Chapter 25
(a) [C|-]i ([Crji + [Rli) = [Cl"]2,
[cr]i([cr]¡ + 3.0) = (o.io)2 => [cn¡ = 0.003 33 M
=> [Cl"]o/[Cr]i = 0.10/0.003 3 = 30
(b) Using [Cl"]0 =1.0in(a)givcs[Cr]o/[Cl"]i = 1.0/0.30 - 3.3
(c) As [Cl"]0 increases, thc fraction of [CI"]¡ increases.
25-9.
The sum of anion charge in the spreadsheet is -0.001 59 M, and the sum of cation
charge is 0.002 02 M. Either some of the ion concentrations arc inaccurate, or
there are other ions in the pondwater that were not detected. For example, there
could be large organic anions derived from living matter (such as humic acid from
plants) that arc not detected in this experiment.
A
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
25-10.
Ion
Fluoride
Chloride
Nitrate
Sulfate
B
Formula mass
(g/mol)
18.998
35.453
62.005
96.064
C
D
Concentration
(ug/mL)
(mol/L)
0.26
1.37E-05
43.6
1.23E-03
5.5
8.87E-05
12.6
1.31 E-04
E
Ion
charqe
-1
-1
-1
-2
Sum of anion charae =
Sodium
Ammonium
Potassium
Magnesium
Calcium
22.990
18.038
39.098
24.305
40.078
2.8
0.2
3.5
7.3
24.0
1.22E-04
1.11 E-05
8.95E-05
3.00E-04
5.99E-04
Sum of cation charge =
F
Charqe
(mol/L)
-1.37E-05
-1.23E-03
-8.87E-05
-2.62E-04
-0.00159
1
1
1
2
2
1.22E-04
1.11E-05
8.95E-05
6.01 E-04
1.20E-03
0.00202
(a) The hydrophilic stationary phase is a zwitterion with fixed positive and
negative charges. Anions arc retained by positive charges and cations are
retained by negative charges. In hydrophilic interaction chromatography, the
stationary phase is polar and there is thought to be a thin layer of aqueous
phase on the surface of the stationary phase. Solvent must be made more polar
to compete with thc stationary phase to elute polar solutes. Eluent strength is
increased when thc acetonitrile content is decreased.
Chromatographie Methods and Capillary Electrophoresis
329
(b) Eluent strength increases in hydrophilic interaction chromatography as the
fraction of aqueous phase increases. With 20 vol% acetonitrile / 80 vol%
aqueous buffer, the eluent strength is high and both ions are eluted rapidly,
without an opportunity to be separated. The eluent strength of 40 vol%
acetonitrile / 60 vol% aqueous buffer is lower, so thc ions are eluted slower
and more selectively.
25-11.
Hydrophobic regions of the protein arc less soluble in water as the salt
concentration in the water increases. This decrease in solubility of nonpolar
substances in water with increasing salt concentration is known as "salting out."
By decreasing thc salt concentration, the protein becomes more soluble in the
aqueous phase and can be eluted from the column. Eluent strength increases as the
salt concentration decreases.
25-12.
At pH 2 (0.01 M HCl), TCA is more dissociated than DCA, which is more
dissociated than MCA. The greater the average charge of the compound, thc more
it is excluded from the ion-exchange resin and the more rapidly it is eluted.
25-13.
(a) Sodium octyl sulfate dissolved in the stationary phase forms an ion-pair with
NE or DHBA. Other ions in the eluent compete with NE or DHBA, and
slowly elute them from the column by ion exchange.
(b) Construct a graph of (peak height ratio) vs. (added concentration of NE). The
.r-intcrccpt gives [NE] = 29 ng/mL.
Added NE siqnal
0.298
0
0.414
12
0.554
24
0.664
36
0.792
48
330
Chapter 25
0.8
07
y = 0.01032x +0.29680
0.6
0.5
0.4
Intercept
= -28.8
0.2 ' |
0.1 •!
-50
-40
-30
'"•"
-20
r
-10
Q't*
0
i
10
"i
20
i
30
t .........,
40
50
Added NE (ng/mL)
25-14.
This is an example of indirect detection. Eluent contains naphthalcnetrisulfonate,
which absorbs at 280 nm. Charge balance dictates that when one of the analyte
anions is emerging from the column, there must be less naphthalcnetrisulfonate
anion emerging. Since analytes do not absorb as strongly at 280 nm, the
absorbance is negative withrespectto the steady baseline.
25-15.
(a) K+ in reservoir = (0.75)(1.5 L)(2.0
[7—1)(2 J
K Z W
= 4.5 mol
Flow rate = (20 x 10"3 mol LKOH )(0.0010¿) - 2.0x10-5 mol KOH
min
Time available =
2.25 x IQ^min
60 mm h
4.5 mol K
mol KOI I
2.0 x IO"5
min
= 2.25 x 105min
= 3.8 x 102 h
(b) A flow of 5.0 mM KOH at 1.0 mL/min provides
(5.0 x 10- 3mol KOH/L)(0.00l 0 L/min) = 5.0 x IO"6 mol KOH/min
5.0 x 1 Piniol KOH/min
ft
„„
,Ä .
s/miñ
= 8.33 x 10'8 mol KOH/s
One electron provides one OH" at the cathode, so the current must provide
8.33 x 10-8 mol eVs. We multiply by the Faraday constant to convert moles of
electrons into coulombs:
(8.33 x IO"8 mol e7s)(9.648 5 x1o4 C/mol e")
= 8.0 x 10-3 c / s . g 0 x 10-3 A = 8 0 mA
60
To produce 0.10 M KOH at 1.0 mL/minrequires20 times as much current,
because the concentration of KOH is 20 times higher than 5.0 mM. Thc
current at the end of the gradient will be (20)(8.0 mA) = 160 mA = 0.16 A.
331
Chromatographie Methods and Capillary Electrophoresis
25-16.
Vent
\
Î
Cathode
\
H 2 0)+ 20H- ^
CH3SO3"
reservoir:
with 2 M
Controlled
current
power
supply
2H 2 0 + 2 e - - ^
NH4+CH3S03"
Anion-exchange
barrier membrane
Pump
H20in
• ^ y\£
CH3S03H
generation
chamber
25-17.
V^T
Pt anode
H20->
2H+ 1/i02(g) + 2e"
\
Liquid
Liquid
out
in
t
I
à
1
0 2 slripper
c
Cation
trap
CH3S03H+
out
CH3SO3H + Vt02(g)
(a) There is a range in which retention volume is logarithmically related to
molecular mass. The unknown is compared to a series of standards of known
molecular mass.
(b) FM IO5 is near the middle range of thc 10 pm pore size column.
25-18.
(a) Vt = 71(0.80 cm) 2 (20.0 cm) = 40.2 mL
VT-VQ
(b) KM - i/ m _r/ 0 25-19.
25-20.
27.4-18.1
35.8-18.1 "
U,5i
Ferritin maximum is in tube 22 (= 22 x 0.65 mL) = 14.3 mL = Vo
Ferric citrate maximum is in tube 84 (= 84 x 0.65 mL) = 54.6 mL = Vm
Does this value of Vm make sense? The total column volume is Vx = TIT2
= ii(0.75 cm)2(37 cm) = 65.4 mL, so Vm = 54.6 mL is plausible.
20.8- 14.3
Transferrin maximum = tube 32 = 20.8 mL => A av - 54 6 _ 143 - 0.10
X
(a) The vertical line begins at log (molecular mass) = 3.3 => mass = 103-3 =
2 000 Da.
(b) A vertical line at 6.5 mL intersects the 10-nm calibration line at
log (molecular mass) » 2.5 => mass = IO2-5 - 300 Da.
length
332
25-21.
Chapter 25
(a) The total column volume is Tir2 x length = TC(0.39)2 (30) = 14.3 mL. Totally
excluded molecules do not enter the pores and are eluted in the solvent volume
(the void volume) outside the particles. Void volume = 40% of 14.3 mL = 5.7
mL.
(b) The smallest molecules that completely penetrate pores will be eluted in a
volume that is thc sum of the volumes between particles and within pores =
80%ofl4.3mL=11.5mL.
(c) These solutes must be adsorbed on thc polystyrene resin. Otherwise, they
would all be eluted between 5.7 and 11.5 mL.
25-22.
A graph of log (molecular mass, MM)
Vs. VT should be constructed.
log(MM) Kr(mL)
aldolase
5.199
35.6
catalase
5.322
32.3
ferritin
5.643
28.6
thyroglobulin
5.825
25.1
BlucDextmn
6.301
17.7
unknown
?
30.3
y = -0.0631x+7.416
<5 6.0
.*> 5.8
«? 5.6
V, (mL)
15
20
25
30
35
40
The equation of thc graph ofKav vs. log (MM) is y = -0.063 1 x + 7.416. Inserting
x = 30.3 gives y = log (MM) = 5.50 ==> molecular mass = 320 000
25-23.
Electroosmosis is thc bulk flow of fluid in a capillary caused by migration of the
dominant ion in the diffuse part of the double layer toward the anode or cathode.
25-24.
At pi I 10, thc wall of the bare capillary is negatively charged with -Si-O" groups
and there is strong electroosmotic flow toward the cathode. At pH 2.5, the wall is
nearly neutral with —Si—Oil groups and there is almost no electroosmotic flow.
The few -Si-O" groups left give slight flow toward the cathode. The
aminopropyl capillary also has positive flow at pH 10, but the rate is only about
half as great as that of the bare capillary. The negative charge might be reduced
because there are fewer -Si-O" groups (because some of them have been
converted to -SÍ-CH2CH2CH2NH2) or because some of thc aminopropyl groups
are protonated (-Si-CH2CH2CH2NH£ ) at pH 10. At pH 2.5, all the aminopropyl
groups arc protonated. The net charge on thc wall is positive and thc flow is
reversed.
Chromatographie Methods and Capillary Electrophoresis
25-25.
333
Arginine is the only amino acid listed with a positively charged side chain. All of
thc derivatized amino acids have a negative charge because the fluorescent group
and the terminal carboxyl group are both negative. Arginine is least negative, so its
electrophoretic mobility toward the anode is slowest and its net migration toward
the cathode (from electroosmosis) is fastest.
25-26.
Under ideal conditions, longitudinal diffusion is the principle source of zone
broadening. Even under ideal conditions, thc finite length of the injected sample
and, possibly, the finite length of the detector contribute to zone broadening.
In real electrophoresis, adsorption on the capillary wall and irregular flow paths
due to imperfections in the capillary could contribute to zone broadening. For an
experimental study of zone broadening, see D. Xiao, T. V. Le, and M. J. Wirth,
"Surface Modification of the Channels of Poly(dimcthylsiloxane) Microfluidic
Chips with Polyacrylamide for Fast Electrophoretic Separations of Proteins," Anal.
Chem. 2004, 76,2055.
25-27.
(a) At pH 2.8, electroosmotic flow will be very small. Anionic analyte will
migrate from negative to positive polarity with little effect from the reverse
electroosmotic flow.
(b) Thc conductivity of thc buffer needs to be higher than the conductivity of the
sample so that the sample will stack. At lower buffer concentration, analyte
bands will be broader and resolution of heparin from its impurities would be
diminished.
(c) High buffer concentration gives high conductivity, high current, and high heat
generation. The narrow column reduces the current and the heat generation
and makes it easier to cool the entire volume inside thc capillary.
(d) Li+ has lower mobility than N a \ so the conductivity of lithium phosphate
solution will be lower than the conductivity of sodium phosphate solution at
the same pH. The lower the conductivity, the higher the electric field required
to generate thc same current.
High field strength reduces the migration time to shorten the analysis. Also,
according to Equation 25-14, the number of plates increases in proportion to
applied voltage.
334
25-28.
Chapter 25
(a) Volume = cross-sectional area x length
100 x 10-9 cm3 (= lOOpL) = (12 x io-4 c m )(50 x 10-4 cm)(length)
=> length = 0.167 mm
(b) Time = distance/speed
Width of injection band (in seconds) = At
=
bandlen
fit"
speed
=
0-167 mm
24 m m / 8 s
0.055 7 s
^injection = A/A/Ï2 = 0.016 s
(c) ^diffusion = ^2Dt = V2(1.0x 10- 8 m 2 /s)(8s) = 0.00040 s
( d ) CTtolal = adiiTu2sion + trinjc?tion= (0.004 s) 2 + (0.016 s) 2
=>CTtotal= 0.016 s
w =
4atoia\ - 0.064 s
25-29.
Electroosmotic flow can be reduced by (a) lowering the pH, so the charge on the
capillary wall is reduced; (b) adding ions such as +H3NCH2CH2CH2NH£ that
adhere to the capillary wall and effectively neutralize its charge; and (c) covalently
attaching silanes with neutral, hydrophilic substituents to the Si—O* groups on thc
walls. A cationic surfactant can form a bilayer, such as that shown in Figure 25-24,
which effectively reverses the charge on the wall.
25-30.
25-31.
In the absence of micelles, neutral molecules are all swept through the capillary at
the electroosmotic velocity. Negatively charged micelles swim upstream with
some electrophoretic velocity, so they take longer than neutral molecules to reach
thc detector. A neutral molecule spends some time free in solution and some time
dissolved in the micelles. Therefore, the net velocity of the neutral molecule is
reduced from the electroosmotic velocity. Because different neutral molecules
have different partition coefficients between the solution and the micelles, each
type of neutral molecule has its own net migration speed. Wc say that micellar
electrokinetic chromatography is a form of chromatography because thc micelles
behave as a "stationary" phase in the capillary because their concentration is
uniform throughout the capillary. Analyte partitions between the mobile phase and
the micelles as thc analyte travels through thc capillary.
(a) Volume of sample = cross-sectional area x length
= «^(length) = 7i(25 x 10" 6 m) 2 (0.0060m) = 1.18 x i o " ' m 3
335
Chromatographie Methods and Capillary Electrophoresis
¿P
=
128n¿t(Vol" me )
'
¡no*
128(0.001 0 kg/(m-s))(0.600 m)(1.18xlQ-H m3)
(4.0 s)7i(50x 10-6 m)4
= 1.15 x 10 4 Pa (= 1.15 x 104kg/(m-s2))
.
AP
(b) AP = Apg => h = — =
1.15xlQ4kg/(m-s 2)
3
(1 000kg/m )(9.8m/s2) "
u / m
Since thc column is only 0.6 m long, wc cannot raise the inlet to 1.17 m.
Instead, we could use pressure at the inlet (1.15 ^ 10 4 Pa = 0.114 atm) or an
equivalent vacuum at the outlet.
25-32.
(a) Volume = ^ ( l e n g t h ) = TC(12.5 X 10-6 m )2 ( 0 .0060 m) = 2.95 x 10"'2 m3 =
2.95nL. Moles = (10.0 x 10-6M)(2.95 X 10-9 L) = 2 9.5 fmol.
(b) Moles injected = p a p p U ~ teflC = Mapp [¿J ~ J
^ C
In order for the units to work out, we need to express the concentration, C, in
mol/m3: (10.0 x 10-6 m o |/L)(l 000 L/m3) = 1.00 x IO"2 mol/m3
(moles)/-t(Ks/Kb)
Papp tm^C
(29.5 x 10-'5mon(0.600m)(l/10)
=
8
2
(3.0 x 10- m /(V>s))(4.0 S)TI(12.5 x 10-6 m )2(j.00 x 10'2 mol/m3)
- 3.00 x 103 V
25-33.
16 r2
16 (6.08 min)2
__ l A d . .
Electrophoretic peak: N - - y = { ¿ m min)í
- 9.2 x IO4 plates
4I.7(VwQ.|)2
Chromatographic peak: N ~ (A/B+1.25)
41.7(6 0 3 ^ ^ 0 3 7 min)2 _
(1.45 + 1.25)
]Q,
(According to my measurements, both plate counts are about 1/3 lower than thc
values labeled in the figure from thc original source.)
25-34.
(a) Fumarate is a longer molecule than maléate, so we guess that fumarate has a
greater friction coefficient than maléate. Electrophoretic mobility is
(charge)/(friction coefficient). Both ions have the same charge, so we predict
that maléate will have the greater electrophoretic mobility.
(b) Since maléate moves upstream faster than fumarate, fumarate is eluted first.
(c) Since the anions move faster than the endosmotic flow, the faster anion
(maléate) is eluted first.
336
25-35.
Chapter 25
(a) pH 2: , W r a l = p ^ = (,. 3 x , 0 - 8 ^ j ( ^ O P l Y j
=
^
x |(H
^
Migration time - (0.52 m)/(5.66 x 10-4m/s) = 9.2 x JO2 s
PH 12: »neutra, =PeoE = (s.l x 1 0 - » ^ ) ( ^ 0 ¿ ^ = 3 .5 3 * 10'3 m/s
Migration time = (0.52 m)/(3.53 x 10-3 m/s) = 1.47 x io 2 s
(b) pH2: p a p p = pep + p e o = (-1.6+ 1.3) x lO" 8 ^- = -0.3 x 10-8 —
V-s
V'S
The anion will not migrate toward the detector at pH 2.
pH 12: papp - p e p + p e o = (-1.6 + 8.1) x l O ' 8 ^ = 6.5 x 1 0 ' 8 ^
V*s
V-s
»anion = Papp* = (ó.5 x 10« ^ J ( ^ ^ j
- 2.8 3 x 10"3 m/s
Migration time = (0.52 m)/(2.83 x IO'3 m/s) = 1.84 x io 2 s
25-36.
(a) Thc net speed of an ion moving through the capillary by electroosmosis plus
electrophoresis is proportional to electricfield(u„et = p app £), which, in turn, is
proportional to voltage. Increasing voltage by 120 kV/28 kV - 4.3 should
increase the speed by 4.3 and decrease the migration time by 4.3. Peak I has a
migration time of 211.3 min at 28 kV and 54.36 min at 120 kV. Theratiois
211.3min/54.36min = 3.9.
(b) Plate count is proportional to voltage (N = i i g - j - ). Increasing voltage by
a factor of 4.3 should increase the plate count by 4.3.
(c) Bandwidth is proportional to the \h¡Ñ (N = ¿ d 2 /a 2 => a = ¿jA/Ä) .
Increasing voltage by 4.3 should increase N by 4.3 and decrease bandwidth by
1A/43 = 0.48. Bandwidth at 120 kV should be 48% as great as bandwidth at
28 kV.
(d) Increasing voltage makes the ions move faster, which gives them less time to
diffuse apart. Therefore, the bandwidth isreducedand resolution is increased.
337
Chromatographie Methods and Capillary Electrophoresis
25-37.
At low voltage (low electric field), the number of plates increases in proportion to
voltage, as predicted by Equation 25-14. Above -25 000 V/m, thc capillary is
probably overheating, which produces band broadening and decreases the number
of plates.
140000
. 100000
E
o/
g
60000
Q-
40000
o
70
80000
o
re
o
/ o
120000
#
Electric field (V/m)
20000
10000
0 •'
25-38.
N =
5-55 f?
w
i72
20000
30000
40000
5,55 (39.9 min)2
= 1.35 x IO4 plates
(0.81 min)2
Plate height = 0.400 m/(1.35 * IO4 plates) = 30 pm
25-39.
/ =
=
«net
v (' = migration time, L = length, u = speed, E = field)
Papp^
L
LIE . _.
LIE
=> Mapp = ^ = 7 7 T 2 f o r C I " andM-app =: 17.78 t o r l
Therefore, we can write that the difference in mobilities is
LIE
LIE
Apapp(l-Cl) = T7T2 - "¡778 ( ¿ / ^ is an unknown constant)
But wc know that Apapp(I-Cl) = [p eo + uep(I")] - [peo + Mep(Cl*)] =
Pep(l-) - Mep(C1") = ° 0 5 * 10 " 8 m 2 / ( s ' V ) i n T a b , e
For the difference between Cl" and B r we can say
„„
LIE
LIE
Apapp(Br-Cl) = 1 7 J 2 ~ x
XAA
-
and we know that Apapp(Br-Cl) = 0.22 x IO"8 m2/(s-V) in Table 14-1.
Therefore, we can set up a proportion:
LIE _ LIE
Apapp(Br-Cl)
0,22
17.12 " x
Apappd-Cl) " 0.05 - UE_
UE_
17.12 " 17.78
x = 20.5 min
338
Chapter 25
The observed migration time is 19.6 min. Considering the small number of
significant digits in the Ap values, this is areasonablediscrepancy.
25-40.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
A
C
D
I
B
Molecular mass by SDS/capillary g«si electrophoresis
E
Molecular
Migration
Protein
mass (MM) log(MM) time (min)
low
Marker dye
13.17
14200
a-Lactalbumin
4.152
16.46
Carbonic anhydrase
29000
4.462I
18.66
45000
Ovalbumin
4.653
20.16
Bovine serum albumin
66000
4,820
22.36
97000
4.987
Phosphorylase B
23.56
b-Galactosidase
116000
5.064
24.97
Myosin
205000
5.312
28.25
Ferritin light chain
?
17.07
Ferritin heavy chain
?
17.97
F
Relative
migration
time ( M
1.250
1.417
1.531
1.698
1.789
1.896
2.145
1.296
1.364
1/1«.
0.8001
0.7058
0.6533
0.5890
0.5590
0.5274
0.4662
0.7715
0.7329
£ 46
3.4349X +6.889
0.5
0.6
0.7
0.8
0.9
log(MM) = (-3.434 9)//rc| + 6.889 = 4.239 for tnl = 1.296 (ferritin light chain)
= 4.372 for tK\ • 1.364 (ferritin heavy chain)
lo M
Molecular mass = 10 ß( M) = 17 300 (ferritin light chain)
= 23 500 (ferritin heavy chain)
Molecular masses observed from amino acid sequences are 19 766 and 21 099 Da
339
Chromatographie Methods and Capillary Electrophoresis
25-41
F
E
C
D
B
A
|
jpn/po)-l
Charge (Az) Migration Apparent Electro1 Protein charge ladder
phoretic
time (s)
j mobility
relative to
2
mA2/(Vxs) mobility
native
3 Total length
pn
protein
4 of column
343.0 6.27E-08 -7.02E-09
0.00
5 Lt(m) =
o
-9.20E-09
0.31
355.4
6.05E-08
-1
0.840
6
0.61
5.84E-08
-113E-08
368.2
-2
Distance
to
7
5.63E-08
-1.34E-08
382.2
0.92
-3
8 detector
1.18
-1.53E-08
395.5
5.44E-08
-4
9 Ld (m) =
5.26E-08
-1.71
E-08
1.44
409.1
-5
0.640
10
1.72
5.06E-08
-1.91
E-08
424.9
-6
11 Voltage (V) =
4.90E-08
-2.07E-08
1.94
438.5
-7
25000
12
453.0 4.75E-08 -2.22E-08
2.17
-8
13 Field (V/Lt) =
2.37
467.0 4.60E-08 -2.37E-08
-9
2.98E+04
14
-10
482.0 4.46E-08 -2.51 E-08
2.57
15 Migration time of
-11
496.4 4.33E-08 -2.64E-08
2.76
16 neutral marker (s) =
-12
510.1 4.22E-08 -2.75E-08
2.93
308.5
17
3.09
524.1 4.10E-08 -2.87E-08
-13
18 Electroosmotic
A
3.23
-14
536.9 4.00E-08 -2.97 E-08
19 mobility (m 2/(Vxs)) =
3.38
-3.07E-08
551.4
3.90E-08
-15
6.97E-08
20
3.81
E-08
-3.16E-08
3.51
565.1
-16
21
577.4 3.72E-08 -3 25E-08
3.63
-17
22 A20 =ÍA107A17VA14
3.73
-3.32E-08
588.5
3.65E-08
-18
23 D5 = ($A$10/C5)/$A$14
24 E5 = D5-$A$20
slope from points 0 to -2 = -3.27874
25 F5 = (E5/$E$5)-1
slope from points 0 to -3 = -3.28115
26 F25 = SLOPE(B5:B7,F5: F7)
slope from points 0 to -4 = -3.36122
27
0
Slope from -3
to 0 = -3.28
-2
-A
-6
AZn
-8
-10
-12
-14
-16
-18
-20
0.00
1.00
2.00
(]Wpo) - 1
3.00
4.00
The graph is curved, most likely because thc shape and friction coefficient of thc
protein change somewhat as the degree of acctylation increases. The first 4 points
(from JC = 0 to JC = -3) lie on a straight line with a slope of-3.28. This slope is the
340
Chapter 25
charge of thc unmodified protein, ZQ = -3.28. There is no reason why z0 should be
an integer. At any given pH, such as pH 8.3 in this experiment, the native protein
is likely to have a fractional average charge because of different amounts of
ionization in different species that arc all in equilibrium.
25-42.
S05f:
p c p = -8.27 x 10-8 m2/(s-V) in Table 14-1
Kapp • Peo + Pep = 16.1 x 10-8 - 8.27 x 10-8 = 7 g 3 x JQ-8 m 2/( s . V )
Br:
Mep = -8.13 x 10-8 m 2/( s . V ) in Table 14-1
Uapp = Peo + Pep = 16.1 x I O"8 - 8.13 x 10"» = 7.97 x 10-8 m 2/( s . V )
Hav = 2 Ph
+ 7.97 * IO"8) = 7.90 x 10« m2/(s-V)
Ap = (8.27-8.13) x 10-8 = 0.14 x 10- 8 m 2 /(sV)
N = \4(Resolution)^J
= [4 ( 2 . 0 ) ~ j
= 2.0 x 1 n5 plates
25-43.
In the absence of micelles, thc expected order of elution is cations before neutrals
before anions: thiamine < (niacinamide + riboflavin) < niacin. Since thiamine is
eluted last, it must be most soluble in thc micelles.
25-44.
Carbon atoms labeled with black circles in cyclobarbital and thiopental arc chiral,
with four different substitutents. These compounds arc not superimposablc on their
mirror images. Thc carbon atom indicated by the diamond in phénobarbital is not
chiral because two of ils siibstituents arc identical. Cyclodcxtrin has a chiral
pocket, in which these compounds can bind. The equilibrium constant for
association of each of the cnantiomers of cyclobarbital and thiopental with
cyclodcxtrin will not be thc same. Each cnantiomer spends a different fraction of
time associated with cyclodcxtrin as it migrates through the capillary. Therefore,
cyclobarbital and thiopental will each separate into two peaks. Phénobarbital will
only give one peak because it docs not have cnantiomers.
ÇH,
°<VN^0
^V
NH C
Cyclobarbital
H
H
£>V Q?/
Thiopental
H
Phénobarbital
341
Chromatographie Methods and Capillary Electrophoresis
25-45.
(a) Plate height rises sharply at low velocity because bands broaden by diffusion
when they spend more time in the capillary. This is thc effect of the B term in
the van Deemter equation, and it always operates in capillary electrophoresis.
Plate height rises gradually at high velocity because solutes require a finite
time to equilibrate with the micelles on thc column. This is thc effect of the C
term in the van Deemter equation, and it is absent in capillary electrophoresis
but present to a small extent in micellar clcctrokinetic capillary
chromatography.
(b) There should be no irregular flow paths because the micelles arc nanosized
structures in solution. The large A term most likely arises from extra-column
effects, such as the finite size of the injection plug and thc finite width of the
detector zone.
25-46.
For the acid H2A, the average charge is a H A . + 2a A2 ., where a is the fraction in
each form. From our study of acids and bases, we know that
K|[H + ]
K\K2
a
==
2
2 ==
2
HA[H+] + K\[W] + KiK2
"A " [H>] + Ki[H+] + K,K2
where K\ and K2 are acid dissociation constants of H2A. The following
spreadsheet finds the average charge of malonic acid (H2M) and phthalic acid
(H2P) and finds that the maximum difference between them occurs at pH 5.55.
arge Difference Between Malonic and Phthalic: Acids
1
2
3
4
S
6
7
8
9
10
11
12
13
14
15
A
Malonic:
Kl =
1.42E-03
K2 =
2.011--06
Phthalic:
Kl 1.12E-03
K2 =
3.90E-06
»
pH
5.52
5.53
5.54
5.55
5.56
5.57
5.58
5.59
c
[H+l
3.OE-06
3.OE-06
2.9E-06
2.8E-06
2.8E-06
2.7R-06
2.6E-06
2.6R-06
D
Alpha
HM0.600
0.594
0.589
0.583
0.577
0.572
0.566
0.561
i;
Alpha
M20.399
0.405
0.410
0.416
0.421
0.427
0.433
0.438
1
Alpha
HP0.436
0.430
0.425
0.419
0.413
0.408
0.402
0.397
m
SAS3*C3/(C3A2>SA$3*C3+$AS3*$AS5)
E3 $A$3*$AS5/ÍC3A2+$A$3*C3 s A S V ^ A ^ i
F3 «= $AS8*C3/(C3A2+$A$8*C3 *$A$8*SA$10)
C3 - $AS«*SASI0/(C3A2+$AS14*C3-($AS8*$^ .StO)
G
Alpha
P20.563
0.569
0.574
0.580
0.585
0.591
0.597
0.602
1
J
H
Charge
Average charges
Malonate Phthalate Difference
-1.398
-1.562 -0.16392
•1.403
-1.567 -0.16405
-1.409
-1.573 -0,16413
-1.415
-1.579 -0.16418
-1.420
-1.584 -0.16417
-1.590 -0.16413
-1.426
-1.596 -0.16404
-1.432
-1.601
-0.16391
-1.437
C3 = 10 A - i 3
H3 = -D3 2*i:3
13 -F3-2 *G3
J3 -I3-H3
342
~e
A*,
¿s-4/.
Chapter 25
f v
W
]Q
_
a -
_
A/A
(JC//?)+ [H+]
-
A
K +
R[H+]
Approximating tx as l 6 a, we can write
K
_
K
Aa
K+[\i+]~ K+R\\i+}
V¿"
.im
\¡K+[H+]
Act
(/;- nATTH^i
V/r+fUM
+
+
Vrî " (If + [H ])(K + R[H ])
^
(/? i W X w i
VÂ T7ÏFÎ (K + R [H+])
7
(b) The function - p has thc form - where u and v are functions of [H + ]:
u = (R-]ys¡K[H+]
v = ^K+[H+)(K
¿fa
(?)
f/v
rf h
-V-7777IT+W
1 he derivative of »/v is _, r i , , , =
,
¿
d [tv\
v
Setting thc derivative equal to zero gives
du
dv
_du
-Vd[H+] + "d[H+]-°
The derivatives are
du
d[W]
-J^~
=
=>
+ R[H+])
t
—-
V
dW]=UdW)
dv
(A)
¡—
(R~lNK
= (K + R[H+]){iK + [H+])-!/2 + \/A-+ [H+] (R)
Inserting u and v and the two derivatives into Equation A gives an equation
that can be solved for [H+].
V
{d^
=u
{d^]j
\/A'+[H+](A'+/?[Ht]){(Ä
1)V^}
= (R- l)\/^[H+]{A'+/i[H+]>5(A'+[H+])-l'2 +
Solving for [H+] gives — after many lines of algebra —
_ K + K-JYTZR
[H+] 2R
(c) Setting R = 1 gives [H+] =
log[H + ] = -log K- log 2
pH = PÀ--0.30
K +
^
9
=
1K
\/A-+[H+](/?)}
Chromatographie Methods and Capillary Electrophoresis
25-48.
343
(a) In ion mobility spectrometry, gaseous ions arc generated by in-adiating analyte
plusreagentgas (such as acetone in air) with high energy electrons (ß
emission) fromradioactive63Ni. Periodically, ions arc admitted into a drift
tube by a short voltage pulse applied to an electronic gate (a grid). In the drift
tube, ions experience a constant electric field that causes either cations or
anions to migrate from thc gate to a detector at the other end of the tube. The
time to reach the detector is thc drift time. Ions drift at a constant speed
governed by the driving force of the electric field and thc retarding force of
friction (drag) by the atmosphere of gas (usually dry air) in the drift tube.
Also, gas in the drift tube flows from the detector to thc source, further
decreasing the migration speed of an ion.
Thc electric field in ion mobility spectrometry causes ions to migrate
from the source to the detector, just as the electric field in electrophoresis
causes ions to migrate. Drift time in ion mobility spectrometry is the same
quantity as migration time in electrophoresis. Thc mobility of an ion in liquid
or in gas is governed by the chargc-to-size ratio. The greater the charge and
the smaller thc size, the greater thc mobility. In liquid or gas, the retarding
force is caused by collisions with solvent or gas molecules.
(b)
E
F
D
C
A
B
1 Ion Mobility Spectrometry
2
N
Wifi (s)
Volts
td(S)
3 k=
5.0000
2.68E-01
1.94E+03
100
4 1 38065E-23 J/K
0.5000
8.47E-03
1.94E+04
1000
5 e=
2000
0.2500 2.99E-03 3.87E+04
6 1.60218E-19 C
3000
0.16671 1.63E-03I 5.80E+04
7 T =
0.1250 1.06E-03 7.73E+04
4000
300 K
8
5000
0.1000 7.59E-04 9.64E+04
9 H=
0.0833 5.78E-04 1.15E+05
6000
0.00008 mV(sV)
10
7000
0.0714 4.60E-04Î 1.34E+05
z
=
11
0.0625 3.77E-04 1.52E+05
8000
12
1
9000
0.0556 3.18E-04 1.70E+05
13 L =
10000
0.0500 2.72E-04 1.87E+05
0.2 m
14
0.0417 2.10E-O4 2.19E+05
12000
15 »9 =
14000
0.0357 1.69E-04 2.47E+05
s
5.00E-05
16
0.03131 1.41 E-04 2.71E+05
16000
16kT(ln2)/e/
=
17
0.0278 1.22E-04 2.90E+05
18000
18 2.86707E-011
20000
0.0250 1.07E-04 3.03E+05
19
A
20 D4^SA$14 2/($A$10*C4
A
21 E4 = SQRT($ A$16 2+($A $187C4)*CM1*2)
A
22 F4 = 5.55*(D< /E4) 2
Chapter 25
4.0E+05
3.0E+05
•n
E
i 2.0E+05
B
«S
K
1.0E+05
!<, = 0.2 ms
O.OE+00 V
0
5000
10000
Volts
15000
20000
Increasing V increases N by decreasing the drift time, and therefore decreasing
thc time for diffusion to broaden thc peak. Increasing the time that the ion
gate is open increases the initial width of thc peak, and therefore decreases the
plate number. The peak can never be narrower than the pulse that is admitted
by the gate. At high voltage, the effect of/g on plate number overwhelms the
effect of t& The disadvantage of using a short gate opening time is that fewer
ions arc admitted to thc drift cell and the signal will be weaker.
(c) Decreasing T increases N because diffusional broadening decreases with
decreasing temperature.
(d) N = 5.55 (tdlw]/2)2
= 5.55(0.024 925s/0.000 154 s) 2 = 1.45 x IO5 plates
Theoretical wfa = t2 +
{£,")%
, „ e „ (\ 6(1.38 x 10-23 J/KK300 K) ln2^
ftfi
= (5.0 x .0-5 s) 2 + [ ( 1 ^ 0 0 V ) ( L 6 O 2 / l o . , 9 c ; ( 1 ) J ( 0 . 0 2 4 9 25 s) 2
= 1.674 x 10-8 s 2
Theoretical N = 5.55 (/d2Avi/22) = 5.55 (0.024 925 s)2/(1.674 x 10-8 s 2)
= 2.06 x 105 plates
CHAPTER 26
GRAVIMETRIC ANALYSIS, PRECIPITATION TITRATIONS, AND
COMBUSTION ANALYSIS
26-1.
(a) In adsorption, a substance becomes bound to the surface of another
substance. In absorption, a substance is taken up inside another substance.
(b) An inclusion is an impurity that occupies lattice sites in a crystal. An
occlusion is an impurity trapped inside a pocket in a growing crystal.
26-2.
An ideal gravimetric precipitate should be insoluble, easilyfilterable,pure, and
possess a known, constant composition.
26-3.
High relative supersaturation often leads to formation of colloidal product with a
large amount of impurities.
26-4.
Relative supersaturation can be decreased by increasing temperature (for most
solutions), mixing well during addition of precipitant, and using dilute reagents.
Homogeneous precipitation is also an excellent way to control relative
supersaturation.
26-5.
Washing with electrolyte preserves the electric double layer and prevents
peptization.
26-6.
HNO3 evaporates during drying. NaN03 is nonvolatile and will lead to a high
mass for the precipitate.
26-7.
During thc first precipitation, thc concentration of unwanted species in the
solution is high, giving a relatively high concentration of impurities in the
precipitate. In the reprecipitation, the level of solution impurities is reduced,
giving a purer precipitate.
26-8.
In thermogravimetric analysis, thc mass of a sample is measured as the sample is
heated. The mass lost during decomposition provides some information about
the composition of thc sample.
26-9.
A quartz crystal microbalance consists of a specially cut, thin, disk-shape slice of
quartz with gold electrodes on each of the two faces. Application of an
oscillating electric field causes the crystal to oscillate at a characteristic
frequency. Binding of small masses to the gold electrodes increases thc mass of
345
346
Chapter 26
thc system and lowers thc oscillation frequency. From the change in frequency,
wc can deduce how much mass was bound.
*< i«
26_10
'
0-2146 g AgBr
187.772 g AgBr/mol
D i
[NaBr] =
IM
26-11.
=
U 4 2 9
* l0_3
1.142 9 x 10-3 mol
50.00 x 10-3 L =
0.104 g Cc0 2
l72.114gCc0 2 /mol
mo1 A
0022
êBr
86M
= 6 0 4 3 x 1<H mo1 Ce
° 2 - 6.043 x |0-4 m o l Ce
= 0.084 66 g Ce
. . „, „
0.084 66 g
weight % Ce = 4 J ? ñ x 100 = 1.94 wt%
26-12,
Formula mass of AgCI = 107.8 + 35.4 = 143.2
iA m
0.088 90,«-Agq
rn0lAgCI =
l43.2^^geiVmdAgCI = 6 ' 2 0 8 < * 10 " 4 ™*
, J.
6.208] x 10-4 mef-CT
m0,radlum =
2 jw^eirtnol Ra " = 3.1040 x i0-4 mol
3.104o x 1<H mol RaCl 2 = J ) R Q a 9 ' , 9 / 2 g , y ' ,
* .r g RaCl2/mol RaCI2
_
0.091 92gRaCI
X
" 3.104o * IO"4 mol RaCI2 = 2 9 6 h 8 R a C l 2 / m o 1
RaCl2
formula mass of RaCl2 = atomic mass of Ra +2(35.4 g/mol) = 296.13 g/mol
=> atomic mass of Ra = 225.3 g/mol
26-13.
One mole of product (206.240 g) comes from one mole of piperazine (86.136 g).
Grams of piperazine in sample =
(0.712 9 g of piperazine/g of sample) x (0.050 02 g of sample) = 0.035 66
w
r
^206.240^
Mass of product = [ 8 6 J 3 6 j (0.035 66) = 0.085 38 g.
26-14.
2.500 g bis(dimclhylglyoximate) nickel (II) = 8.653 2 x 10-3 m o | Ni =
0.507 85 g Ni = 50.79% Ni.
26-15.
Formula masses: CaC| 4 H|o0 6 -H 2 0 (332.32), CaC0 3 (100.09), CaO (56.08). At
550°, CaC|4H|n06-H 2 0 is converted to CaC03 (calcium carbonate).
332.32 g of starting material will produce 100.09 g of CaO.
Gravimetrie, Precipitation, and Combustion Analysis
347
Mass at 550° = (100.09/332.32)(0.635 6 g) = 0.191 4 g. At 1 000°C, the product
is CaO (calcium oxide) and the mass is (56.08/332.32)(0.635 6 g) - 0.107 3 g.
26-16.
2.378 mg C0 2 / (44,010 g/mol) = 5.403 3 x IO 5 mol C0 2 = 5.403 3 x 10-5 m o i c
= 6.4900 x 1 0 4 g C
ppm C - IO6 (6.4900 x 10-4/6.234) =104.1 ppm
26-17.
2.07% of 0.998 4 g = 0.02067 g of Ni = 3.521 x IO"4 mol of Ni.
Thisrequires(2)(3.521 x 10-4) mol of DMG - 0.081 77 g.
A 50.0% excess is (1.5)(0.081 77 g) = 0.122 7 g. The mass of solution containing
0.122 7 g is 0.122 7 g DMG/(0.021 5 g DMG/g solution) = 5.705 g of solution.
The volume of solution is 5.705 g/(0.790 g/mL) = 7.22 mL.
26-18.
Moles of Fc in product (FC2O3) = moles of Fe in sample.
Because 1 mole of (Fe203) contains 2 moles of Fe, wc can write thc equation
1
f ^ | ^
¡
= 3.306 x 10 -3 mol of F..
This many moles of Fe equals 0.919 2 g of FeS04 • 7 H 2 0. Because we analyzed
just2.998goutof22.131 g of tablets, the FeS0 4 - 7 H 2 0 in the 22.131 g sample
is Y^f
(0.919 2 g) = 6.786 g. This is the FeS04 • 7 H 2 0 content of 20 tablets.
The content in one tablet is (6.786 g)/20 = 0.339 g.
26-19.
(a) Mass of product (CaC03) - 18.546 7 g - 18.231 1 g = 0.315 6 g
™ 1 C a C ° 3 - Goo.087 g/mol) - 3.153 x 10-3 mol
Thc product contains 3.153 mmol Ca = (3.153 x 10-3 mol)(40.078 g/mol) =
0.1264 g Ca,
0 2 64fiCa
wt%Ca
rt¿ J4,g mineral, x 100 = 19.98%
wi/o v,» = 0 632
(b) The solutions are heated before mixing to increase thc solubility of thc
product that will precipitate. If the solution is less supersaturated during the
precipitation, crystals form more slowly and grow to be larger and purer than
if they precipitate rapidly. The larger crystals are easier to filter.
(c) (NH4)2C204 provides oxalate ion to prevent CaC204 from redissolving.
Also, the ammonium and oxalate ions provide an ionic atmosphere that
prevents thc precipitate from peptizing (breaking into colloidal particles).
348
Chapter 26
(d) AgN03 solution is added to the filtrate to lest for Cl- in the filtrate. If Cl" is
present, AgCl(.v) will precipitate when Ag + is added. The source of Cl" is thc
HCl used to dissolve thc mineral. All the original solution needs to be
washed away, so no extra material is present that would increase the mass of
final product, which should be pure CaC03(.s).
26-20.
(a) 70 kg [
k^
J = 441 g P in 8.00 x l o3 L. This corresponds to
441 g P
8.00 x 103L " ° 0 5 5 ' ^L
or 5 5
(b) Fraction of P in one formula mass is
'
3
m
g/10°
mL
-
595 4 ¿ = 1.722%.
P i n 0 . 3 3 8 7 g o f P 2 O 5 - 2 4 M o O 3 = (0.01722)(0.3387) = 5.834 mg
This is near thc amount expected from a dissolved man.
26-21.
Let * = mass of NH 4 C1 and y = mass of K2CO3 .
For thc first part, 1/4 of thc sample (25 mL) gave 0.617 g of precipitate
containing both products:
mol NH.CI
1
mol K2C03 x 2
I5T492J ( 337 - 2? )
+
[ñik\) <358-33)
Y
V
ß «MW H4
g (fuBK
=
0.617 g
'
($ = phenyl = CíH 5 )
We multiplied moles of K 2 C 0 3 by 2 because one mole of K 2 C 0 3 gives 2 moles
of <|J 4 BK. In the second part, half of thc sample (50 mL) gave 0.554 g of <J>4BK:
mol K2CO) x 2
2 ( n j è r j <358-33)
^
=
°-554g
=> ^ = 0.213 7 g •• 14.5wt%K 2 COî
"gíÍBK-
Putting this value ofy into thc first equation gives JC = 0.215? g = 14.6 wt%
NH 4 C1
26-22.
Fe 2 0 3 + A1 2 0 3
Tor^T
"eat»
Fc + Al 2 0 3
T774 g
349
Gravimetric, Precipitation, and Combustion Analysis
The mass of oxygen lost is 2.019 - 1.774 = 0.245 g, which equals 0.015 31 moles
of oxygen atoms. For every 3 moles of oxygen there is 1 mole of Fe 2 03, so
moles of Fc 2 0 3 = \ (0.015 31) = 0.005 105 mol of Fe 2 03- This much Fe 2 03
equals 0.815 g, which is 40.4 wt% of the original sample.
26-23.
Let JC = g of FeS0 4 • (NH 4 ) 2 S 0 4 • 6H 2 0 and y = g of FeCl2 * 6H 2 0.
We can say thatx+y • 0.548 5 g. The moles of Fe in thc final product (Fe 2 03)
must equal the moles of Fc in the sample.
The moles of Fe in Fc 2 0 3 = 2 (moles of Fe 2 0 3 ) = 2 m g j p j = 0.002 1016 mol.
Mol Fe in FeS0 4 • (NH 4 ) 2 S 0 4 • 6 H 2 0 = JC / 392.13 and
mol Fe in FcCl2 * 6H 2 0 - y 1234.84.
(1)
0.002 1 0 1 6 = 3 9 ^ 7 3 + 234^84
Substituting x = 0.548 5-y into Eq. (1) gives y = 0.411 46 g of FeCl2 • 6H 2 0.
MassofCl = 2 ( f f o f ) (0.41146) = 0.12423 g = 22.65 wi%
26-24.
(a) Let JC = mass of AgN0 3 and (0.432 1 -JC) = mass of Hg 2 (N0 3 ) 2 in unknown.
Each mol of AgN03 gives I/3 mol Ag3[Co(CN)ö] and each mol of Hg2(N03)2
gives 1/3 mol (IIg2)3[Co(CN)6h. Mass of both products must equal 0.451 5 g:
mol Ag 3 Co(CN) 6
T Í - )
3M69.873>
mol(Hg 2 ) 3 [Co(CN) 6 ] 2
(538-643) +
^
v
_v_
mass of Ag 3 Co(CN) 6
••
3^
•
S
525.19
'
0M3.62) - 0.4515
^
mass of ( Hg 2 ) 3 [Co(CN) 6 ) 2
=> JC = 0.173 l g = 40.05wt%
(b) 0.30% error in 0.451 5 g = ± 0.001 35 g. This changes thc equation of (a) to:
538 3 +
2
1 633 62)
KT6^)
^ ) K ltT^)
'
=0.451 5 (+0.001 35)
1.056 952 JC + 0.448 020 - 1.036 844 JC = 0.451 5 (±0.001 35)
0.020 109 x = 0.451 5 (±0.001 35) - 0.448 020
0.020 109 .r = 0.003 480 (±0.001 35)
0.003480(±0.001 35)
0.003480(±38.8%)
n ,„
x
=
0.020 109
0.020 109
= 0.17 g ± 39%
350
26-25.
Chapter 26
(a) Balanced equation for overall (31.8%) mass loss:
31.8% mass loss
^
Y2(OH)5C1-JCH20
FM 298.30+ x(18.015)
(2 +x)( 18.015) + 36.461 =
*
,mass lost
Y2O3 +
JCH 2 0 + 2 H 2 0
FM 225.81
FM (2+.v)(18.0l5))
( 0 . 3 1 8 ) [ 2 9 8 . 3 0 + JC(18.015)]
••
v
31.8% of original mass
•
=>
+ HCl
FM36 >4 6I
JC-
1.82
(b) Logical molecular units that could be lost are 1120 and HCl. At - 8 . 1 % mass
loss, thc product is Y2(OH)5CI. Loss of 2 more H2O would give a total mass
loss of
1.82H20 + 2H 2 0
68.82
Y2(OH)5Cl- 1.82H20 == 331.09 == 2 0 - 8 ° / o
Loss of HCl from Y2(OH)sCI would give a total mass loss of
1.82H2Q > HCl
69.25
Y2(OH)5Cl- 1.82H20 ~ 331.09 == 209%
The composition at the -19.2% plateau could be cither Y202(0H)C1
(from loss of 2H 2 0) or Y 2 0(0H) 4 (from loss of HCl).
, , «,,
. v
W
massofKPOT
_
118.070 3
"" maS8 0fK(DJH,.r)2P04 ~ 136.085 3 + 2.012 55JC
a
Cross-multiply: (136.085 3)a + (2.012 55) cue - 118.070 3
(2.012 55)ca - 118.070 3 - (136.085 3)a
Divide by (2.012 55)a:
X
118.070 3
(136.085 3)q
=
" (2.012 55)a " (2.012 55)a
x
"
58.667 0
a
~ 67.618 3
For fully deuteratcd material, JC = 1 and a = J +57 618 3 • 0.854 976
58.667 0
(b)
x =
0.856 77 - 67.618 3 = 0.856 3 2
(c) For thc function JC =_/(a), we can write
ex =
For*
58.667 0
a
. _ , . „ . OF
58.667 0
- 67.618 3 , — = - — - 2 — g , v i n g
If 58.667 0 V ~
58.667 0 eu
351
Gravimetrie, Precipitation, and Combustion Analysis
(d) Fore« « 0.000 1, ex =
(58.667 0)(0.000 1)
J
=
(0 .856 77)
°
D:H stoichiometry = x±ex
\fexwere0.001,
= 0.856±0.008
(58.667 0)(0.001)
thene x =
(0.856 77)2
nfW
0 0 8
=
nno
°08
and
D:H stoichiometry = 0.86 ± 0.08
26-27.
(a) Formula mass of YBa2Cu307_jc = 666.19-(16.00) JC
34.397 mg
mmol 0fYBa2Cu3O7_x in experiment - r 6 6 6 1 9 _(i6.00)x]mg/mmol
(34.397-31.661) mg
mmol of oxygen atoms lost in experiment j 6 QQ m g / m m o ]
= 0.171 00 mmol
From the stoichiometry of the reaction, wc can write
mmol oxygen atoms lost
3.5 -x
mmol YBa2Cu307_x
1
0.17100
34.397/[666.19-(16.00)«] "" "
* =* X "
9fUu , ¿ w ¿
(without regard to significant figures)
(b) Now let the uncertainty in each mass be 0.002 mg and let all atomic and
molecular masses have negligible uncertainty.
The mmol of oxygen atoms lost are:
r34.397(±0.002) - 31.661(±0.002)]mg
16.00 mg/mmol
=
2.736(±0.002 8)
16.00
- 0.171 00 (±0.102%)
Thc relative error in the mass of starting material is 34397
=
0.005 8%
Thc master equation becomes
0.171 00 (±0.102%)
,_
34.397 (±0.005 8%)/[666.19 - (16.00) JC]- = 3.5-JC
0.171 00 (±0.102%)[666.19 - (16.00)*] -- (3.5 - JC)[34.397 (±0.005 8%)]
113.918 (±0.116)- [2.736 (±0.002 79)] JC
= 120.389 5 (±0.006 98) - [34.397 (±0.002)] JC
[31.66 (±0.003 46)] * - 6.471 5 (±0.116)
- 0.2044 (±1.79%) = 0.204 ± 0.004
352
Chapter 26
r
26-28.
H0 2 C
J
DTPA
k.
CO, M
Neutral DTPA has 2 carboxylic acid protons and 3 ammonium protons. Wc arc
not given the pA'a values, but, by analogy with EDTA, we expect carboxyl pKa
values to be below ~3 and ammonium pKa values to be above ~6. At pH 14, we
expect all thc acidic protons of DTPA to be dissociated, so thc predominant
species will be DTPA*-. At pH 3-4, thc nitrogen atoms should all be protonated,
but the carboxyl groups should be all (or mostly) deprotonatcd. The predominant
species is probably DTPA2".
For HSO4, pK„ = 2.0. At pH 14 and at pH 3, sulfate should mainly be in thc
form SO4".
At pH 14, DTPA5- is apparently a strong enough ligand to chelate Ba 2+ and
dissolve BaS0 4 (s). At pH 3-4, DTPA2- is not a strong enough ligand to dissolve
BaS04(¿). Another way to say thc same thing is that H+ at a concentration of
10"3-104 M competes with Ba2+ for binding sites on DTPA, but H + at a
concentration of IO 1 4 M does not compete with Ba 2+ for binding sites on DTPA.
26-29.
In combustion, a substance is heated in the presence of excess O2 to convert
carbon to C 0 2 and hydrogen to 1120. In pyrolysis, the substance is decomposed
by heating in thc absence of added 0 2 . All oxygen in the sample is converted to
CO by passage through a suitable catalyst.
26-30.
WO3 catalyzes thc complete combustion of C to CO2 in thc presence of excess
O2. Cu converts SO3 to SO2 and removes excess O2.
26-31.
The tin capsule melts and is oxidized to Sn02 to liberate heat and crack the
sample. Tin uses thc available oxygen immediately, ensures that sample
oxidation occurs in thc gas phase, and acts as an oxidation catalyst
26-32.
By dropping the sample in before very much O2 is present, pyrolysis of the
sample to give gaseous products occurs prior to oxidation. This minimizes thc
formation of nitrogen oxides.
353
Gravimetric, Precipitation, and Combustion Analysis
26-33.
C 6 H 5 C0 2 H + 7 O 2
FM 122.123
—
7 CO2 + 3 H 2 0
44.010
18.015
One mole of C6H5CO2H gives 7 moles of C 0 2 and 3 moles of H2O.
4.635 mg of benzoic acid - 0.037 95 mmol, which gives 0.265 7 mmol CO2
(= 11.69 mg CO2) and 0.113 9 mmol H 2 0 ( = 2.051 mg H 2 0).
26-34.
C 8 H 7 N0 2 SBrCl + 9^ 0 2 — 8CO2 + f H 2 0 + \ N 2 + S 0 2 + HBr + HCl
26-35.
100 g of compound contains 46.21 g C, 9.02 g H, 13.74 g N, and 31.03 g O. The
atomic ratios are C : H : N : O =
46.21 g
9.02 g
13.74 g
31.03 g
:
:
12.010 7 g/mol 1.007 94 g/mol 14.006 74 g/mol ' 15.999 4 g/mol
= 3.847 : 8.949 : 0.9810 : 1.940
Dividing by the smallest factor (0.981 0) gives the ratios C : H : N : O = 3.922 :
9.12 : 1 : 1.978. The empirical formula is probably C 4 HoN0 2 .
26-36.
C 6 H|2
+
C2H4O
FM 84.159
- • CO2 + H 2 0
44.053
44.010
Let x = mg of C ö H \ 2 and y = mg of C2H4O
JC + y = 7.290.
We also know that moles of CO2 = 6 (moles of CóH 12) + 2 (molesof C2H 4 0),
by conservation of carbon atoms.
21.999
44.010
t8TÏ59j
l44.053j
Making thc substitution JC - 7.290 - y allows us to solve for y.
6
+ 2
=
y = 0.767 mg = 10.5 wt%.
26-37.
The atomic ratio H : C is
( 6.76 ±0.12 g ^
U007 94g/moU
6.707 + 0.119
Í71.17 + 0.41 g\ - 5.926±0.0341
=
6.707 ± 1.78%
5.926±0.576% "
,
U J i ± u
....
'wl
Il2.0l0 7g/molj
If we define thc stoichiometry coefficient for C to be 8, then the stoichiometry
coefficient for H is 8(1.132 ± 0.021) = 9.06 ±0.17.
The atomic ratio N:C is
354
Chapter 26
( 10.34 ±0.08 g ^
4.006 74 g/molj
g/molj
U14.006
71.17 + 0.41 R \
12.010 7 g/molj
í
0.738 2 ± 0.005 7 _ 0.738 2 ± 0.774%
5.926 + 0.0341 " 5.925 ± 0.576%
= 0.1246 + 0.0012
If we define the stoichiometry coefficient for C to be 8, then the stoichiometry
coefficient for N is 8(0.1246 ± 0.001 2) = 0.996 8 + 0.009 6.
Thc empirical formula is reasonably expressed as C8H9,06±0.l7No.997±o.010
26-38.
The reaction between 1 l2S0 4 and NaOH can be written
H 2 S0 4 + 2NaOH -^ 2H 2 0 + Na 2 S0 4
One mole ofH2S0 4 requires two moles of NaOH. In3.01 mLof0.01576M
NaOH there arc (0.003 01 L)(0.015 76 mol/L) = 4.744 x \0-S m o | 0 f NaOH.
The moles of H2S0 4 must have been (Vt)(4.144 x I0"5) • 2.372 * I 0 5 mol.
Because one mole of H 2 S0 4 contains one mole of S, there must have been
2.372 x IO"5 mol of S (= 0.760o mg). The percentage of S in the sample is
0.7606 mg S
6.123 mg sample x , 0 ° " 12.4 wt%.
26-39.
(a) Experiment 1 : JC = 10.16o pmol CF s = 2.707 pmol Cite
95% confidence interval = Jt±~7=
(2.262)(2.707)
= 10.16o ±
-T™
=10.16o±1.93 6 pmolClExperiment 2: x = 10.770 pmol Cl"
ts
95% confidence interval = * ± - p
= 10.77o ±
s = 3.20s pmol CF
(2.262)(3.205)
"7=
= 10.77o±2.29 3 pmolCl-
/5|2(/7|-|) + S22(»2-1)
/2.707 2 (10-l) + 3 . 2 0 5 2 ( 1 0 - l j
¿pooled - • ' \ /
„,+„, 7
-\J
10+ 10 2
= 2.966
/calculated •
1*1-*2 I
^ ^
^
/ mn2
+
| 10.16p-10.77p|
^ - "
/(lO)dO)
~J^~ -\¡^OTl0
» 0.46o < ttabulated for 18 degrees of freedom for
95% confidence level (or even for 50% confidence level)
Therefore, thc difference is not significant. The result means that addition of
355
Gravimetrie, Precipitation, and Combustion Analysis
excess CF prior to precipitation does not lead to additional coprecipitation of
CF under the conditions of these experiments. (In general, under other
conditions we would expect extra CF to lead to extra coprecipitation.)
(c) 10.0mgofSO 24 = 0.10410 mmol - 24.2g5 mg BaS0 4
(d) In Experiment 1, the precipitate includes an additional 10.16o umol Cl =
5.08 pmol BaCl2 = 1 .05R mg BaCI2. The increase in mass is
(1.058)/(24.295) = 4.35%. This represents a large error in thc analysis.
26-40.
(i) r(cxcess) + Ag + -* Agl(i)
[Ag+] = Ksp (for Agi) / [I"]
(ii) A stoichiometric quantity of Ag+ has been added that would be just
equivalent to I", if no Cl" were present. Instead, a tiny amount of AgCI
precipitates and a slight amount of 1" remains in solution.
(iii) Cl-(excess) + Ag+ — AgCl(s)
[Ag+] = Ksp (for AgCI) / [CF]
(iv) Virtually all I" and Cl" have precipitated.
[Ag + ]«[CI] ^[Ag+]=^Ksp
(for AgCI)
(v) There is excess Ag' delivered from the buret.
/"volume added past 2nd equivalence pointa
[Ag+] = [Ag + ] titranl - ^
total volume
J
26-41.
At Ve, molesof Ag+ = moles of 1"
(FemL)(0.051 1 M) = (25.0 mL)(0.082 3 M) => Ve 40.26 m l .
WhenFA g+ = 39.00 mL, [F] = ^ Q ^
9
'
0 0
(008230) (25.offi39.OO,
= 1.006 x 10-3 M, [Ag+] = Kspl[V] = 8.3 x 1 0 l 4 M => pAg+ = 13.08.
When VAg+ = Ve, [Ag+][1'] = JC2 =A.sp =>jr = [Ag+] = 9.1 x IO*9 M
=>pAg+ = 8.04.
When ^Ag+ = 44.30 mL, there is an excess of (44.30 - 40.26) = 4.04 mL of
{
4 04
^\
Ag+. [Ag+] -[ 2 5.00V44.30j(O051 10) = 2.98 x 10-3 M ^ p A g + = 2 .53.
26-42.
At thc equivalence point, [Ag+][F] = Ksp => (x)(x) = 8.3 x 10"17
=> [Ag+] = 9.1 x IO 9 M. The concentration of CF in the titration solution is thc
initial concentration (0.0500 M) corrected for dilution from an initial volume of
40.00 mL up to -63.85 mL at the equivalence point:
[Cl] = (0.0500 M ^ ^ T ^ J • 0.031 3 M
356
Chapter 26
Is AgCI solubility exceeded? The reaction quotient is Q = [Ag+][CF] = (9.1 x
10-9)(0.031 3) = 2.8 x 10-10, which is greater than Ksp for AgCI (= 1.8 x IO 10 ).
Therefore, AgCI begins to precipitate before Agi finishes precipitating. If [CF]
were - 2 times lower, AgCI would not precipitate prematurely.
26-43.
Moles of Ca 2+ = moles of C2O4"
(Fc)(Û\025 7M) = (25.00 mL) (0.031 1 M) => Ve - 30.25 mL
(a) Thc fraction of C204~ remaining when 10.00 mL of Ca 2 ' have been added is
(30.25 - 10.00)/(30.25) = 0.669 4.
[C2Or)¡] = (0.669 4)(0.031 10 M) ( f j ^ ) = 0.014 87 M
[Ca2+] = Kspl[C2024] = (1.3 x 10»)/(0.01487) = 8.7 x IO"7
=> pCa2+ = -log(8.7 x 10-7) = 6.06
(b) At thc equivalence point, there are equal numbers of moles of Ca 2+ and
C2O4 dissolved. Call each concentration JC:
[Ca2+] [C2024-] = (x)(x) = Ksp ^ JC = sjk7p = 1.1 4 * 10*4 M
pCa 2+ =-log(l.l 4 x io-t)= 3.94
(c) [Ca 2+ ] = (0.025 70 M) ( 3 5 ' ^ " o f f " 2 5 ) = 0.002 035 M.
26-44.
pCa 2+ = 2.69
Equilibrium constants for ion pair formation:
[AgX(ag)]
[Agf][X ]
=
<
10331 (X = CI)
IO46 (X = Br)
IO66 (X = I)
Calling thc ion pair formation constant Äff, we can write
[AgX(aq)] = ATffAg+][X-]. But the product [Ag+][X*] is just Ksp. So,
[AgX(aq)] = KfKsp. Putting in thc values Ksp = IO*9-74 for AgCI, IO"12-30 for
AgBr, and IO*16-08 for Agi gives
[AgCl(a<?)] = 103 3110-9.74 = 10-6.43 M = 370 nM
[AgBiiaq)] = 104610"l2-30 = 1 0 - 7 - 7 0 M = 20 nM
[Agl(aq)] = 106-610-1608 = 10-9.48 M = 0.33 nM
26-45.
FM of NaCI = 58.443. FMofKBr = 119.002. 48.40 mL of 0.04837 M
Ag + = 2.341 1 mmol. This must equal thc mmol of (CI"+ Br"). Let JC = mass
of NaCI and y = mass of KBr. JC + y = 0.238 6 g.
357
Gravimetric, Precipitation, and Combustion Analysis
58.443
119.002
mol Cl"
mol Br"
= 2.341 1 x IO"3 mol
Substituting JC = 0.238 6 -y gives y - 0.2000 g of KBr = 1.681 mmol of KBr
= 1.681 mmol of Br = 0.1343 g of Br = 56.28% of the sample.
26-46.
mmol of BrCH2CH2CH2CH2Cl = , 7 j
46 mg/*mol
- 0.4822 mmol
There will be 0.482 2 mmol of Cl" and 0.4822 mmol of Br" liberated by reaction
with CH30"Na+.
. *, w.
+
Ag required for Br" =
0.482 2 mmol
,_„. .
1 8 7 6 m L
0 < 0 2 5 70 mmoF/mL ~
Thc same amount of Ag + is required to react with Cl", so the second equivalence
point is at 18.76+18.76 = 37.52 mL.
26-47.
M+
+
X" ^
Analyte
Titrant
r ° \P
C° V
Mass balance for M:
Mass balance for X:
MX(s)
c£, V°u = [M + ](F^+ Vx) + mol MX(s)
C^VX = [X-](F^+ Vx) + mol MX(i)
Equating mol MX(s) from both mass balances gives
4 C - tM+K<i+ vx> - ¿x vx - W<
a(c°M-
+v
x)
[M+] + [X-^
which can be rearranged to VY = K. ——
M
^C°. + [M+]-[X-]J
26-48.
Your graph should look like the figure in the text.
26-49.
Mass balance for M: C^ VM = [M m+ ] (VM + F°, ) + jc{mol MxXm(s)}
Mass balance for X: C°, F £ = [X*~] (F"M + F^) + wfmol M ^ í ^ í ) }
Equating mol MvXffJ from the two equations gives
ï & V **i<»M+'S » = = < 'S -[X"] <(,M+'S»
which can be rearranged to the required form.
358
26-50.
Titration of Chromate with Ag+:
A
1
2
K*«
3
4
CM =
5
6
Cx =
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
vx =
B
C
pM
1.2E-12
D
[M]
3.47E-06
3.98E-06
5.46
5.4
E
F
G
H
I
vM
[X]
9.98E-02
7.57E-02
0.013
1.932
C 2 = 10*-B2
D2 = $A$2/C2 A 2
E2 = SA$8*{2*$A$6+C2-2*D2)
/($A$4-C2+2*D2)
0.1
5.3
5.2
5.01 E-06 4.78E-02
6.31 E-06 3.01 E-02
5.342
8.717
0.1
5.1
5
7,94 E-06 1.90E-02
1.00E-05 1.20E-02
11.734
14.195
10
4.9
4.8
4.6
4.4
4
3.6
3.2
2.8
2.4
2.3
2.2
2.1
2
1.9
1.8
1.26E-05 7.57E-03
1.58E-05 4.78E-03
2.51 E-051 1.90E-03
3.98E-05 7.57E-04
1.00E-04 1.20E-04
2.51 E-04 1.90E-05I
6.31E-04 3.01 E-06
1.58E-03 4.78E-07
3.98E-03 7.57E-08
5.01 E-03 4.78E-08
6.31 E-03 3.01 E-08
7.94E-03 1.90E-08
1.00E-02 1.20E-08
1.26E-02I 7.57E-09
1.58E-02I 4.78E-09
16.057
17.388
18.908
19.564
19.958
20.064
20.189
20.483
21.244
21.583
22.020
22.589
23.333
24.321
25.650
1
e
1
CD
1
ol
0
—^
5
10
15
20
25
Volume of Ag* (mL)
26-51.
Consider the titration of C+ (in aflask)by A" (from a buret). Before thc
equivalence point, there is excess C+ in solution. Selective adsorption of C+ on
the CA crystal surface gives the crystal a positive charge. After the equivalence
point, there is excess A" in solution. Selective adsorption of A" on the CA crystal
surface gives it a negative charge.
Gravimetric, Precipitation, and Combustion Analysis
26-52.
359
Beyond the equivalence point, there is excess Fc(CN)£ in solution. Selective
adsorption of this ion by thc precipitate will give the particles a negative charge.
26-53.
A known excess of Ag + is added to form Agi (s). In the presence of Fe 3+ , the
excess Ag+ is titrated with standard SCN" to precipitate AgSCN(j). When Ag+ is
consumed, SCN" reacts with Fe 3+ to form the red complex, FeSCN2+.
26-54.
50.00 mL of 0.365 0 M AgN03 = 18.25 mmol of Ag+
37.60 mL of 0.287 0 M KSCN = 10.79 mmol of SCN"
Difference = 18.25- 10.79 = 7.46 mmol of I" = 947 mg of F
CHAPTER 27
SAMPLE PREPARATION
27-1.
There is no point analyzing a sample if you do not know that it was selected in a
sensible way and stored so that its composition did not change after it was taken.
27-2.
"Analytical quality" refers to the accuracy and precision of the method applied to
the sample that was analyzed. High quality means that the analysis is accurate
and precise. "Data quality" means that the sample that was analyzed is
representative and appropriate for the question being asked and that the analytical
quality is adequate for the intended purpose. If an accurate and precise analysis
is performed on an unrepresentative or contaminated or decomposed sample, the
results are meaningless.
27-3.
(a) s2 = s2 + s2 = 3 2 + 4 2 => s0 = 5%.
(b) s2 - s2 - s2 = 42 - 3 2 => i s = 2.6%.
27-4.
mR2 = K¿. m(62) = 36 g => m = 1.0 g
27-5.
Pass the powder through a 120 mesh sieve and then through a 170 mesh sieve.
Sample retained by 170 mesh sieve has a size between 90 and 125 pm. It would
be called 120/170 mesh.
27-6.
11.0 x io 2 g will contain IO6 total particles, since 11.0 g contains IO4 particles.
«KCl -nPm (106X0.01) - IO4.
Relative standard deviation = ^[ñpqlnKa = V(106)(0.01)(0.99)/104 = 0.99%.
27-7.
(a) V00 3 )(0.5)(0.5) = 15.8.
(b) We are looking for the value of z, whose area is 0.45 (since the area from -z
to +z is 0.90). The value lies between z = 1.6 and 1.7, whose areas are
0.445 2 and 0.4554, respectively. Linear interpolation:
z - 1 . 6 _ 0.45 -0.4452
1.7-1.6 - 0,4554-0.445 2 =* Z~]&V(c) Sincez = (x-x)lstx = x±zs = 500±(I.647)(15.8) - 500±26.
The range 474-526 will be observed 90% of the time.
360
361
Sample Preparation
27-8.
Use Equation 27-7, with s s = 0.05 and e = 0.04. The initial value oft for 95%
confidence in Table 4-2 is 1.960. n = fis21 e2 = 6.0 For n = 6, there arc 5
degrees of freedom, so / = 2.571, which gives n - 10.3. For 9 degrees of
freedom, / = 2.262, which gives n = 8.0. Continuing, we find / = 2.365 =>
n = 8.74. This gives / = 2.306 => n = 8.30. Use 8 samples. For 90%
confidence, the initial t is 1.645 in Table 4-2 and the same series of calculations
gives n = 6 samples.
27-9.
(a) mR2 = Ks. For R = 2 and Ks = 20 g, we find m = 5.0 g.
(b) Use Equation 27-7 with ss = 0.02 and e = 0.015. The initial value oft for
90% confidence in Table 4-2 is 1.645. n = t2s2le2 - 4.8
For n = 5, there arc 4 degrees of freedom, so / = 2.132, which gives n = 8.1.
For 7 degrees of freedom, / = 1.895, which gives n = 6.4.
Continuing, wc find t = 2.015 => n = 7.2. This gives t = 1.943 => n = 6.7.
Use 7 samples.
27-10.
A
|
B
|
C
|
¿
1 Evaluation of the relation mR = Ks
2
D
R>
K, = mR2
R%
3 m(pg)
4
57
0.057
0.00325
0.185
0.00476
0.324
0.069
5
68
110
0.049
0.00240
0.264
6
0.223
7
110
0.045
0.00203
0.00123
0.620
0.035
8
506
0.00073
515
0.027
0.375
9
0.00032
0.297
916
0.018
10
0.462
0.00048
955
0.022
11
average
0.344
12
0.141
std dev
13
Average value of Ks
0.34 +0.14 pg
0.7
0.6
0.5
9 0.4
a.
£
0.3
0.2 1
It looks like A's might be
increasing with sample size
0.1
0.0
200
400
600
m(Pfl)
800
' 000
362
Chapter 27
The confidence interval is ±tsl^n, where t is Student's t, s is the standard
deviation, and n is the number of replicate measurements. If« is thc same for all
points, then / is the same for all points and the confidence interval is proportional
to s. The equation in the text is expressed in terms of s. If the confidence
interval is proportional to s, then the same equation should hold for the
confidence interval.
27-11.
(a) Volume = (4/3)nr3, where r - 0.075 mm = 7.5 x 10-3 c m .
Volume = 1.767 x 10^ mL.
Na2CC>3 mass = (1.767 x 10-6 mLX2.532 g/mL) = 4.474 x IO"6 g.
K2CO3 mass - (1.767 x IO'6 mL)(2.428 g/mL) = 4.291 x 10"<> g.
Number of particles of Na2C0 3 - (4.00 g)/(4.474 x 10"<> g/particle)
= 8.941 x 105.
Number of particles of K 2 C03 = (96.00 g)/(4.291 x 10"6 g/particle)
= 2.237 x 10?.
The fraction of each type (which we will need for part c) is
/>Na2CC>3 = (8.941 x 105)/(8.941 x IO5 + 2.237 x 10?) = 0.0384
ÍK2CO3 • (2.237 x 107)/(8.941 x 105 + 2.237 x 10?) = 0.962.
(b) Total number of particles in 0.100 g is n = 2.326 x lO4.
(c) Expected number of Na 2 C03 particles in 0.100 g is 1 /1000 of number in 100
grams = 8.94 x IO2.
Expected number of K2CO3 particles in 0.100 g is 1/1 000 of number in 100
grams = 2.24 x IO4.
Sampling standard deviation = sfñpq = -\j(2.326 x 1 C^XO.OSS 4)(0.962)
= 29.3.
29 3
Relative sampling standard deviation for Na 2 C03 = „ Q . . 2 = 3.28%.
29 3
Relative sampling standard deviation for K2CO3 = - - . ' ^ = 0.131%.
27-12.
Metals with reduction potentials below zero [for the reaction M n+ + we" —»
M(s)] are expected to dissolve in acid. These are Zn, Fe, Co, and Al.
27-13.
HNO3 was used first to oxidize any material that could be easily oxidized. This
helps prevent thc possibility that an explosion will occur when HC104 is added.
Sample Preparation
27-14.
363
Barbital has a higher affinity for thc octadecyl phase than for water, so it is
retained by thc column. The drug dissolves readily in acetone/chloroform, which
elutes it from the column.
27-15.
Cocaine is an amine base. It will be a cation at low pH and neutral in ammonia.
The cation at pH 2 is retained by thc cation-exchange resin. The neutral
molecule is easily elutcd by methanol. Benzoylecgonine has an amine and
carboxylatc functionality. At pH 2, the amine will be protonated and the
carboxylic acid should be neutral, so the molecule will be retained by the cation
exchange column. At elevated pH, the amine will be neutral and thc carboxylate
will be negative. The anion is not retained by the cation-cxchangcr and is eluted
with methanol.
27-16.
The product gas stream is passed through an anion-exchange column, on which
S0 2 is absorbed by the following reactions:
SO2 + H 2 0 — H 2 S0 3
2Resin+OH" + H2SO3 -» (Resin+hSO2^ + H 2 0
The sulfite is eluted with Na2C03/H 2 0 2 , which oxidizes it to sulfate that can be
measured by ion chromatography.
27-17.
Large particle size allows sample to drain through the solid-phase extraction
column without applying high pressure. In chromatography, small particle size
increases thc efficiency of separation, but high pressure is necessary to force
solvent through the column.
27-18.
(a) Solid-phase extraction retains acrylamide while passing many other
components in the aqueous extract of the french fries. We want to remove as
many other components as possible to simplify the chromatographic
analysis. The strong acid of the ion-exchange resin protonates acrylamide
and retains it by ionic attraction:
R-SO3H + CH2=CHCONH2 -> R-SO3 + CH2=CHCONH}
(b) There are many ultraviolet-absorbing components in addition to acrylamide
in the acrylamide fraction obtained from the ion-exchange column.
Ultraviolet absorbance is not specific for acrylamide.
(c) For acrylamide, m/z 72 is selected by thc mass filter Q1 of the mass
spectrometer. This ion dissociates by collisions in Q2. The product m/z 55
Chapter 27
is selected in Q3 for passage to the detector.
CH2=CHCONH^ -> CH2=CHCsO+
m/z 72
m/z 55
CD2=CDCONH£ -> CD 2 =CDC=0 +
m/z 75
m/z 58
(d) Even though many compounds are applied to the chromatography column,
acrylamide is the only one with m/z 72 that gives a significant reaction
product at m/z 55.
(e) Acrylamide gives one peak by selected reaction monitoring of the transition
m/z 72->55. The internal standard gives just one peak for 2H3-acrylamide
monitored by the transition m/z 75->58 with the same retention time as
acrylamide. Thc transition m/z 72->55 does not respond to thc internal
standard, and the transition m/z 75-»58 does not respond to unlabeled
acrylamide. We know the concentration of internal standard added to the
aqueous extract of thc french fries, and we measure the integrated area of the
m/z 75->58 peak for the internal standard. We also measure the integrated
area of the m/z 72-»55 peak for acrylamide. The concentration of
acrylamide in the aqueous extract is found by the proportion
[acrylamide]
[internal standard]
[area of m/z 12-^55 peakl
[area of m/z 75-»58 peak]
(f) With ultraviolet absorption, the internal standard appears at the same elution
time as acrylamide. The molar absorptivity of deuterated internal standard is
probably very similar to that of acrylamide, so equal concentrations of
internal standard and acrylamide contribute approximately the same
integrated area in thc chromatogram. With selected reaction monitoring by
mass spectrometry, thc detector sees either acrylamide or the internal
standard, with no interference from the other, even though they are eluted at
the same time.
(g) The internal standard is mixed with the aqueous extract from the french fries
prior to solid-phase extraction. We expect little isotope effect on the binding
of acrylamide to the solid-phase sorbent or the HPLC stationary phase.
Therefore, the fraction of acrylamide and the fraction of internal standard
that bind to and are recovered from the solid-phase extraction column are
equal. Even though neither one is bound or eluted quantitatively, equal
Sample Preparation
-^63
fractions of each arc bound and eluted. The ratio of acrylamide and internal
standard should remain constant throughout the entire procedure.
27-19.
(a) Highest concentration of Ni ~ 80 ng/mL. A 10 mL sample contains 800 ng
Ni = 1.36 x IO"8 mol Ni. To this is added 50 pg Ga = 7.17 x IO"7 mol Ga.
Atomic ratio Ga/Ni = (7.17 x 10"7)/(1.36 x IO"8) = 53.
(b) Apparently all the Ni is in solution because filtration does not decrease its
total concentration. Sincefiltrationremoves most of the Fe, it must be
present as a suspension of solid particles.
27-20.
One-fourth of the sample (25 mL out of 100 mL) required
(0.011 44 M) (0.03249 L) = 3.717 x IO"4 mol EDTA => (3.717 x 10"4)(4) =
1.487 x 10"3 mol Ba2+ in sample = 0.2042 g Ba = 64.90 wt%.
27-21.
(a) From the acid dissociation constants of Cr(IIl), we see that the dominant
forms at pH 8 are Cr(OH)^ and Cr(OHh(aq). The dominant form of Cr(VI)
is CrOj-,
(b) The anion exchanger retains the anion, CrOj*, but permits the Ci<OH)J
cation and neutral Cr(OF\)3(aq) to pass through, thereby separating Cr(Vl)
from Cr(NI).
(c) A "weakly basic" anion exchanger contains a protonated amine (-+NHR2)
that might lose its positive change in basic solution. A "strongly basic"
anion exchanger ( +NR3) is a stable cation in basic solution.
(d) CrO2," is eluted from the anion exchanger when the concentration of sulfate
in the buffer is increased from 0.05 M in step 3 to 0.5 M in step 4.
27-22.
One possible cost-saving scheme is to monitor wells 8,11,12, and 13
individually, but to pool samples from thc other sites. For example, a composite
sample could be made with equal volumes from wells 1,2,3, and 4. Other
composites could be constructed from (5,6,7), (9, 10), (14, 15,16,17), and (18,
19,20,21). If no warning level of analyte is found in a composite sample, we
would assume that each well in that composite is free of thc analyte. If analyte is
found in a composite sample, then each contributor to the composite would be
separately analyzed. Thc disadvantage of pooling samples from n wells is that
thc sensitivity of the analysis for analyte in any one well is reduced by \ln.
.
•
•
.
.
•
w. H. Freeman and Company
41 Madison Avenue, New York, N.Y. 10010
Houndmills, Basingstoke RG21 6XS, England
C, C. McCorkle, Geo/ogy 2009, 37, 1131
COVER DESIGN: Vicki Tomaselli