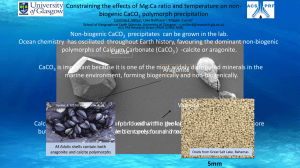
Introduction Calcium carbonate (CaCO3) is a widely used substance for various purposes. It is used as fertilizer and a filler and pigment material in paper, plastics, rubbers, paints, and inks but also in pharmaceutics, cosmetics, construction materials, and asphalts and as a nutritional supplement in animal foods(Martinez,2002).Just like many minerals calcium carbonate is also one of the substances that are mined. It is mined from limestone and it is processed to size for use in different applications (Crapper, 2012) There are three polymorphs calcium carbonate: vaterite, aragonite and calcite, what differentiate them is their thermodynamically stability. All these polymorphs can occur at the same time in some environment and conditions Aragonite and calcite are more thermodynamically stable structures, and they most commonly occur in nature (Ratner, 2008).It is important to understand the chemistry of these because Vaterite has been shown to transform to calcite and aragonite in aqueous solution. Experimental evidence has demonstrated that vaterite can transform to aragonite in 60 minutes at 60°C and to calcite in 24 hours at room temperature (Frebida et al , 2021). Even though there is such abundant deposits of limestone on the planet that the possibility of depletion is remote, the application of calcium carbonate depend on the availability particular polymorph. Understanding the behaviour and controlling the growth of the polymorphs of calcium carbonate plays a major role in the synthesis of specific polymorph.in recent times vaterite has been found to be very useful in the in the area of regenerative medicine, drug delivery and a broad range of personal care products. It has been established above that vaterite is very much unstable so knowledge of the composition of the synthesised crystals is important because it will be provide information that can be useful for the manipulation of conditions necessary for the production of the desired polymorph. There are many factors that influence the precipitation of calcium carbonate polymorphs. The most determining factors is the presence of foreign ions or molecules in the solution from which the calcium carbonate precipitate (Frebida et al, 2021). The technique of choice for quantifying these polymorphs of calcium carbonate is X-Ray Diffraction. diffraction occurs when light is scattered by periodic array with long range order producing constructive interference at specific angels (Banerjee, n.d.) .X-Ray Diffraction is a technique for analysing the atomic or molecular structure of materials(esli, 2019). The scattering of light is proportional to the number of electrons around the atom. Diffraction peak is attributed to the scattering from a specific set of parallel planes of atoms, miller indices are used to identify the different planes of atoms( Banerjee,N.D) Therefore, the main objective of this study is to quantify the three polymorphs of calcium carbonate in two samples that were synthesised from a 0.01; & 0.02 M respectively of aqueous solutions of each of Na2CO3 and CaCl2 at different temperature using XRD. Experimential Two precursor materials were used: Sample 5 0.01 M Na2CO3 and 0.02 M CaCl2 at PH OF 8.5 Sample 6 0,O1M Na2CO3, O.O2 PH 6 both sources are analytical grade (Sigma Aldrich ). The synthesized CACO3 was prepared by mixing O.O1M Na2CO3 with 0.02 M CaCL2 at two PH OF 8.5 and 6 respectively . The mixture was left stirring on a magnetic stirrer bar for another 10 minutes before it was filtered with a 0.8 μm membrane filter, then washed thoroughly with distilled water. The powder precipitate was dried in an oven, set at 90℃ overnight and then stored in a blue silica desiccator in darkness X-ray powder diffraction measurements were carried out using a Bruker D8 Advance X-ray diffractometer instrument using Cu Kα radiation. Ideally, the sample should be pulverised to a particle size of less than 20 microns – this is achieved though micronising pulverised powders. This is especially important for quantitative analysis .The powder should be loaded into one of the plastic Bruker sample holders, with the sample identification and depth of at least 1 mm. The surface of the packed powder was pressed and smoothed with a piece of flat glass. Care was taken to ensure that the surface of the sample is flat and level with the top of the sample holder The quantities were sufficient to make a compact sample with correct height and smooth surface. Once the sample was prepared the sample holder was wiped to remove any nonessential sample that is present. After the instrument was switched on the software was started .The commander module of the Bruker AXS model was selected by click on the commander icon. Once the communication between the software was established, Corundum was used for alignment of the goniometer. The diffraction pattern was recorded for 2θ from 0° to 90° and a 2θ step scan of 0.050° was used, counting for 1 s at every step. The total scan time for each sample was 28 min. The voltage and current of the generator were set at 40 kV and 40 mA respectively 4 Results 1.200 RALATIVE INTENSITY 1.000 0.800 0.600 0.400 0.200 0.000 0 10 20 30 -0.200 40 50 2 THETA 60 70 80 90 100 HUNDREDS Difractogram A for sample 5(0.01 M Na2CO3 and 0.02 M CaCl2 at PH 8.5) 1.2000 1.0000 relative intensity 0.8000 0.6000 0.4000 0.2000 0.0000 0 -0.2000 10 20 30 40 50 60 70 2 theta(in degrees) Difractogram B for sample 6 (0.01 M Na2CO3 and 0.02 M CaCl2 at PH 6) 80 90 100 Difractogram C (Fig 1 is the difractograms for pure polymorphs of calcium carbonate) Table of XRD intensities TABLE 1 Polymorphs of Pure calcium polymorphs carbonate intensity Vaterite(25.0 Calcite (29.5 Aragonite(45.9 Sample 5 Sample 6 0.583 no peak at the expected position 1.00 1.0078 no peak at the 0.3388 expected position Calculations for the individual polymorphs for sample 5 %aragonite=(I/Iºarogonite)/ (I/Iºvaterite)+ (I/Iºcalcite)+(I/Iºarogonite) =0/(0.583+1+0)x100 =0% %vaterite =(I/Iºvaterite )/ (I/Iºvaterite)+ (I/Iºcalcite)+(I/Iºarogonite =0.583/ (0.583+1+0)X100 =36.8% %calcite =(I/Iºcalcite )/ (I/Iºvaterite)+ (I/Iºcalcite)+(I/Iºarogonite =1/(1+0.583+0)X100 = 63.2% Calculations for the individual polymorphs for sample 6 %aragonite=(I/Iºarogonite)/ (I/Iºvaterite)+ (I/Iºcalcite)+(I/Iºarogonite) =0.3388/(0.3388+1.0078+0)x100 =25.2% %vaterite =(I/Iºvaterite )/ (I/Iºvaterite)+ (I/Iºcalcite)+(I/Iºarogonite) =(0/(0+1.0078+0.3388) =0% %calcite =(I/Iºcalcite )/ (I/Iºvaterite)+ (I/Iºcalcite)+(I/Iºarogonite) =1.0078/(0+1.0078+0.3388) =74.8% Table 2 concentration of calcium carbonate polymorphs Polymorphs of Sample 5 (PH Sample CaCO3 8.5) 6) %Arogonite 0 25.2 %vaterite 36.8 0 %calcite 63.2 74.8 6(PH Discussions Crystals of calcium carbonate were synthesised using sodium carbonate and calcium chloride. These two samples were prepared at room temperature and at different PH. Table 2 show the results from quantification that was carried on XRD instrument.The result for sample show that the synthesis did not produce arogonite but it is composed by 63.2% of calcite and 36.2 % vaterite. Chen et al. Observed drastic decrease in super saturation during CaCO3 particle synthesis due to short induction times 10 at high pH solutions. For sample 5 it seems as the high Ph of 10 it has the impact in the formation of Aragonite polymorph.Temperature is shown to have impact in the formation of the polymorphs (Tawada, 1987). Suzuki (1991) “The transformed polymorphs are vaterite and calcite at low temperature (14 to 30°C), and aragonite and calcite at high temperature (60 to 80°C)”.Sample 5 results confirm this finding with the formation of vaterite and calcite with the formation of Aragonite. For sample 6 results are not consistent with what other researchers has found. Kojima(2019) found that at PH of 5 to 10 it is possible to synthesize polymorphs of calcium carbonate which contain vaterite but in this current investigation vaterite is not found. Further studies on other thermodynamical properties is needed.