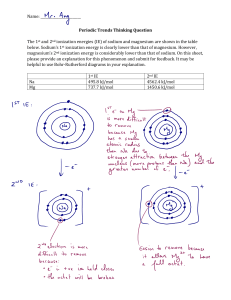
Chemistry IA January 2022 How does changing the concentration of hydrochloric acid affect the rate of reaction of the chemical reaction between magnesium and hydrochloric acid as measured by the production of hydrogen gas? Research Question How does changing the concentration of hydrochloric acid, in the selected range of 2.00 mol/dm3, 1.50 mol/dm3, 1.00 mol/dm3, 0.75 mol/dm3, 0.50 mol/dm3, affect the rate of reaction of the chemical reaction between magnesium and hydrochloric acid as measured by the production of hydrogen gas? Scientific Context When analysing chemical reactions, chemists often observe the rate of reaction. The rate of reaction is defined as the rate of change of the concentration of the reactants or products per unit time. Rate of reaction is a useful tool to control the rate at which a product is produced, therefore the rate of a reaction can be observed and analysed in order to get the highest yield of a chemical reaction and gain as much product as possible. When two particles react, as explained in the Collision Theory, they collide with each other and, by breaking the intermolecular bonds, they create new ones with other particles. In order for a reaction to take place, there must be successful collisions between the particles of the reactants categorised as such if there is enough energy to break the intermolecular bonds and the proper orientation between particles. There are different factors that influence the probability of a successful collision occurring, affecting the rate at which they happen. One of these factors, the one selected to be investigated in this experiment, is the concentration of either of the reactants. Figure 1: graphic portraying how increasing the concentration impacts the number of collisions Hypothesis The rate of reaction can be predicted to increase as the concentration of the hydrochloric acid is increased. This is due to the increase of the number of HCl particles, in a set volume, that are available to make successful collisions with the magnesium ribbon. As more particles are present in a constant volume, they are also closer together allowing for more frequent collisions and a higher probability of a successful collision. The increase of both of these aspects infers an increase in the rate of reaction as the products are formed more rapidly. The rate of reaction can be expected to be directly proportional to the concentration of hydrochloric acid. Choice of Reaction As I was deciding which field to explore for the investigation, I was keen on choosing something which had to do with chemical kinetics which is a very interesting field that allows for extensive analysis of the data gained through a simple experiment. I choose to investigate the following reaction: Mg(s) + 2HCl(l) → MgCl2 (aq) + H2 (g) as it combines my interest in chemistry with the one of biology. MgCl2 is often used in dietary supplements to higher the intake of magnesium into the body, and I take one myself to prevent migraines. Magnesium is part of many metabolic reactions in the body and it is of great interest to pharmaceutical companies to gain the highest possible yield in the optimum conditions as to make the supplements with the least amount of wastage. The factors, like concentration, that impact collisions between particles can be modified to reach a higher yield. Variables: o Independent variable The concentration of HCl will be changed using the values 2.00 mol/L, 1.50 mol/L, 1.00 mol/L, 0.75 mol/L, 0.50 mol/L. Original planning of the experiment programmed a decrease of half in between each concentration, but as the results were inconclusive due to the lack of completion of the reaction and product produced, the concentration range chosen changed to values closer to each other. o Dependent variable The rate of reaction will be measured by observing the time it takes for magnesium to react with hydrochloric acid to produce 50 mL of hydrogen gas. As the concentrations of hydrochloric acid are very low, not all reactions are able to create enough product to fill the glass syringe. 50 cm3 was chosen consequentially to some trial repeats as the optimal measurement of gas produced. The rate of reaction will then be calculated using stoichiometric relationships and with the following formula: 𝑟𝑎𝑡𝑒 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 = ∆ 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 ∆ 𝑡𝑖𝑚𝑒 . o Controlled variables CV# Controlled Variable How it was controlled Why it was controlled CV1 Temperature All solutions and reactions were done and kept at room temperature Changing the temperature impacts how the energy is distributed between the molecules, therefore affecting the rate of reaction CV2 Mass of Mg The amount of magnesium was kept constant throughout the experiment Changing the mass of magnesium means changing the concentration, therefore changing the amount of particles involved in the reaction, affecting the rate of raction CV3 Surface area of Mg The length and amount of pieces of magnesium was Changing the surface area impacts the number of kept constant throughout the experiment molecules exposed to the hydrochloric acid, therefore affecting the rate of reaction CV4 Volume of HCl solution The volume was measured with the same method and kept constant throughout the experiment The ratio between the hydrochloric acid and magnesium molecules would change, therefore affecting the rate of reaction CV5 Solvent Distilled water was used to make all solutions throughout the experiment Different solvents might have different chemical properties, possibly impacting the result of the reaction and reaction rate CV6 Pressure The experiment was conducted at room pressure By changing the pressure the collision rate between particles is changed therefore impacting the rate of reaction CV7 Presence of a catalyst No catalyst was added in any reaction in the experiment Catalysts lower the activation energy of a chemical reaction and therefore speeding up the rate of reaction Equipment and apparatus ● Pipette ● Measuring cylinder (100.00±0.5 mL, 25.00±0.25 mL) ● Glass syringe (100±0.5 cm3) ● Beaker (50.0±7.5 mL, 100±12.5 mL, 250±25 mL) ● Test tubes ● Conical flask (125±12.5mL) ● Stand with a clamp ● Rubber cork for test tubes and conical flask ● Magnesium (Mg) ribbon ● Hydrochloric acid (HCl) (2.00 mol/dm3, 1.50 mol/dm3, 1.00 mol/dm3, 0.75 mol/dm3, 0.50 mol/dm3) ● Ruler (15.00±0.05cm) ● Stop clock (5999.99±0.01”) ● Scissors Figure 2: Glass syringe system connected to conical flask Method 1. The glass syringe was topped with a rubber cork, attached to a silicone tube that ends with another cork in order to create a closed system in order to avoid loss of product in the environment 2. The glass syringe was set up in the clamp on the stand and regulated so that the values on the syringe were easily readable at eye level 3. The hydrochloric acid solution was prepared in a measuring cylinder of 100mL 4. For the solutions that needed to be diluted, the appropriate ratio was chosen and the hydrochloric solution was diluted in distilled water (eg. starting with a 2 mol/L concentration in order to prepare a 1.5 mol/L concentration, the ratio needed is 3:1, diluting 75 mL of HCl solution in 25 mL of distilled water) 5. The solution was then poured into a separate beaker and stirred to avoid a concentration gradient inside the beaker 6. 25mL of the solution was measured in a measuring cylinder using a pipette (CV5) at eye level and then poured into a testing tube 7. 2 pieces measuring 2cm of the magnesium ribbon were cut (CV3, CV4) with a pair of scissors 8. The solution was then poured into the conical flask 9. The magnesium was then added to the solution in the conical flask to start the reaction and the flask was immediately closed with the cork connected to the glass syringe 10. At the same time as closing the conical flask, the start clock was started 11. Observing the glass flask the start clock was stopped once the volume reached 50 mL of hydrogen gas formed 12. Once the volume has been reached the time was recorded and the glass syringe is emptied 13. The conical flask was then emptied into a beaker 14. The conical flask was then rinsed with water 15. The procedure was repeated 4 times for each concentration (2.00 mol/dm3, 1.50 mol/dm3, 1.00 mol/dm3, 0.75 mol/dm3, 0.50 mol/dm3) Safety precautions Source Danger Precautions Magnesium ribbon Flammable The magnesium ribbon was kept away from any source of fire and kept in a protected environment when the needed amount was taken. Only small quantities of the metal were used in the experiment. Hydrochloric acid Corrosive Safety goggles were worn at all times throughout the experiment. Only low concentrations and small volumes of the acid were handled. All equipment was thoroughly washed before touching it with exposed skin. Hydrogen gas Flammable The gas was released from the glass syringe away from any source of fire and any leftover reaction was placed in a fume cupboard so that the gas was not dispersed unsupervised. Only small volumes of hydrogen gas were produced. Other Assistance was used in steps 9 and 10 to avoid any spillage of the acid on the working table. Raw Data Table 1: Time that is taken each trial for the reaction to produce 50 mL of hydrogen gas Time (s) ±0.01 s Experiment number Concentration of HCl (mol/dm3) T1 T2 T3 T4 1 2.00 14.09 13.79 13.23 12.97 2 1.50 18.17 18.76 17.74 17.76 3 1.00 50.85 61.89 51.87 50.85 4 0.75 132.48 95.94 93.19 86.83 5 0.50 357.68 347.01 314.80 370.96 Processing Data In order to identify anomalies in the data set standard deviation was used. If the data was identified to be outside 2 standard deviations from the mean, then the value would be rejected from further calculations. Table 2: Mean and standard deviations of each experimental data set and the accepted range of values Experimental data set Mean (s) 2 standard deviations (s) Accepted range of values (s) 1 13.52 ±1.02 12.50 ≤ 𝑥 ≤ 14.54 2 18.11 ±0.95 17.15 ≤ 𝑥 ≤ 19.07 3 53.87 ±10.74 43.13 ≤ 𝑥 ≤ 64.61 4 102.11 ±41.21 60.90 ≤ 𝑥 ≤ 143.32 5 347.61 ±47.94 299.67 ≤ 𝑥 ≤ 395.55 With the method, no anomalies can be identified following the method as they are all included in the accepted range and all values are kept for calculations and further analysis of the data. Table 3: Average time taken for the reaction to produce 50 mL of hydrogen gas for each concentration of hydrochloric acid used with respective standard deviations Average time (s) Experiment Number Concentration of HCl (mol/dm3) ±0.10 mol/dm3 Value Standard deviation 1 2.00 13.52 ± 0.51 2 1.50 18.11 ± 0.48 3 1.00 53.87 ± 5.37 4 0.75 102.11 ± 20.60 5 0.50 347.61 ± 23.97 Calculation example for the average time: ● Divide the sum of the values by 4 𝑥𝑎𝑣𝑔 = Σ𝑥 4 Uncertainty of the average time ● Calculated with the standard deviation Analysis Table 4: Average time taken for the reaction to produce 50 mL of hydrogen gas for each concentration of hydrochloric acid used with calculated hydrogen gas concentration with respective uncertainties Experiment Number Concentration of HCl (mol/dm3) Concentration of H2 produced (mol/dm3) ± mol/dm3 Time (s) value uncertainty value uncertainty 1 2.00 13.52 ± 0.51 0.50 ±7.07×10-3 2 1.50 18.11 ± 0.48 0.38 ±6.25×10-3 3 1.00 53.87 ± 5.37 0.25 ±5.60×10-3 4 0.75 102.11 ± 20.60 0.19 ±5.34×10-3 5 0.50 347.61 ± 23.97 0.13 ±5.16×10-3 Calculations to find the concentration of hydrogen produced (exemplified with data from experiment 1) Mg(s) + 2HCl(l) → MgCl2 (aq) + H2 (g) ● Calculate the moles of HCl by multiplying the concentration and the volumes used in the experiment (consider the change in units of the volume from cm3 to dm3) and the affiliated uncertainty −3 Eg. moles of HCl 2. 00 𝑚𝑜𝑙 𝑑𝑚 3 × 0. 025 𝑑𝑚 = 0. 05 𝑚𝑜𝑙 ● Calculate the moles of H2 by dividing the moles of HCl by two as observed from the stoichiometric ratio of 1:2 between the two and the affiliated uncertainty Eg. moles of H2 = ● 0.05 𝑚𝑜𝑙 2 = 0. 025 𝑚𝑜𝑙 Calculate the concentration of H2 by dividing the moles by the volume of H2 measured (consider the change in units of the volume from cm3 to dm3) and the affiliated uncertainty 0.025 𝑚𝑜𝑙 Eg. concentration of H2 = 3 0.05 𝑑𝑚 −3 = 0. 50 𝑚𝑜𝑙 𝑑𝑚 Propagation of uncertainty ● For the first two calculations for the concentration of hydrogen, the only uncertainty that is propagated it the one of the volume measurement of the hydrochloric acid −4 ● 3 solution in the measuring cylinder (±0. 25 𝑚𝐿 = ±2. 5 × 10 𝑑𝑚 ) For the calculation of the concentration from the moles of hydrogen and the volume, the following formula is used 2 2 ( ) ( ) 𝑥 ∆𝑥 ∆𝑦 ∆𝑧 = || 𝑦 || + 𝑦 𝑥 Where ∆𝑧 is the uncertainty of the result, ∆𝑥 and ∆𝑦 are respectively the uncertainties of the moles of hydrogen gas and the volume of hydrogen gas Eg. from experiment 1 0.025 ∆𝑧 = || 0.05 || ( −4 2.5×10 0.025 2 ) ( + −4 5×10 0.05 2 ) −3 −3 = ± 7. 07 × 10 𝑚𝑜𝑙 𝑑𝑚 Table 5: concentration of hydrochloric acid and the corresponding rate of reaction of each experiment when 50 cm3 of hydrogen gas was produced Experiment Number Concentration of HCl (mol/dm3) 1 Rate of Reaction (mol/dm3s) value uncertainty 2.00 3.70×10-2 1.49×10-3 2 1.50 2.10×10-2 6.55×10-4 3 1.00 4.64×10-3 4.74×10-4 4 0.75 1.86×10-3 3.79×10-4 5 0.50 3.74×10-4 2.98×10-5 Calculations to find the Rate of Reaction ● To calculate the Rate of Reaction the following formula was used 𝑟𝑎𝑡𝑒 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 = ● ∆ 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 ∆ 𝑡𝑖𝑚𝑒 The two points considered were the initial time (at 0.00 s) and the initial concentration of hydrogen gas (0.00 mol/dm3) and the time it took for the reaction to produce 50 dm3 of hydrogen gas. As the change started from two values of 0, the rate of reaction was calculated by simply dividing the concentration of hydrogen gas by the average time. Eg. from experiment 1 0.50 13.52 𝑟𝑎𝑡𝑒 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 = −2 −3 = 3. 70 × 10 𝑚𝑜𝑙 𝑑𝑚 Propagation of uncertainty ● The uncertainty for each value was calculated using the same formula previously used 2 2 ( ) ( ) 𝑥 ∆𝑥 ∆𝑦 ∆𝑧 = || 𝑦 || + 𝑦 𝑥 Where ∆𝑧 is the uncertainty of the result, ∆𝑥 and ∆𝑦 are respectively the uncertainties of the concentration of hydrogen gas and the average time Eg. from experiment 1 0.50 ∆𝑧 = || 13.52 || ( −3 7.07×10 0.50 2 ) + ( 0.51 2 13.52 ) −3 = 1. 49 × 10 −3 −1 𝑚𝑜𝑙 𝑑𝑚 𝑠 Graph 1; Graph of the concentration of hydrochloric acid against the rate of reaction The trend is shown in the graph shows a linear relationship between the concentration of hydrochloric and the rate of reactions, as one increases the other also increases. Further observations can be made as the graph allows to determine the overall order of the reaction. From the line of best fit shown in the graph, the reaction is likely to be of first order. The R2 value is exactly 1, which indicates a strong positive correlation between the concentration of hydrochloric acid and the rate of reaction. The positive correlation is also observed as there are no points that lie outside of the line of best fit, indicating that the data is quite precise. Conclusion As predicted in the hypothesis, the increase of the concentration of hydrochloric acid caused an increase in the rate of reaction as the amount of particles increases in the set volume (25 mL). There is a difference of 0.037 between the rates of reaction of the lowest and highest concentration, this shows clearly an increase in the rate of reaction and the graph successfully represents the reaction with a strong positive correlation with each other. The rate of reaction is directly proportional to the concentration of hydrochloric acid in the reaction with a linear relationship. Limitations and improvements Limitations Improvement Not all reactions were completed when 50 mL of hydrogen gas were produced. This makes the rate of reaction comparison less precise Conduct more trials to observe the maximum volume of hydrogen produced. Only one point of reference was taken into consideration. Measure the time at different volumes, or measure the volume formed at chosen time intervals. For safety, only very low concentrations were used, allowing for only minimal change in the rate of reaction and making it more susceptible to r. Under safe conditions, use higher concentrations of hydrochloric acid, to observe greater change. The stop clock was manually stopped after the volume reached 50 mL. Especially for faster reactions, the time of reaction of the person stopping is limited, making the accuracy of the time reading limited. The reaction could have been recorded showing the glass syringe and stop clock to then record more accurate data. The temperature of the solution was not measured with a thermometer every time the reaction was carried out but it was kept at room temperature implying that the room was at constant 25ºC. To accurately keep the temperature constant, a water bath measured at 25ºC could have been kept and the temperature could have been measured every time the experiment was to be carried out. Although with 2 standard deviations, no anomalies were identified, there were values in the experiments that were outside of the general trend. - Experiment 1, trial 4 (12.97); experiment 3, trial 1 (61.89), experiment 4, trial 1 (132.48) experiment 5, trial 4 (370.96) These values might have impacted the final result of the investigation. In order to have more accurate data, more trials should have been carried out to have more viable data to substitute the data that does not follow the general trend. The glass syringe apparatus is not a completely closed system and does not assure that no amount of gas is dispersed Assuring the glass syringe is fully empty and all corks are tightly secured to the ends of the syringe before beginning the in the environment. Any uncontrolled amount of gas dispersed alters the accuracy of the reading and the results. experiment. Extensions Possible extensions of this experiment could include ● Manipulating the mass of magnesium; this would be done by changing the amount of magnesium ribbon that is added to the HCl solution. ● Manipulating the surface area of magnesium; this would be done by changing the number of separate pieces the magnesium ribbon is cut in. ● Manipulating the temperature the reaction takes place in; this would be done by heating or cooling down the HCl solution. All these extensions would observe how different factors affect the rate of reaction. In the case of the manipulation of the mass of magnesium, it could be added as an extension to the previous data carried out to find the rate equation and the order in respect of magnesium. This would allow understanding of how the concentration of each reactant impacts the rate of reaction. Bibliography A Gas Syringe Connected by a Rubber Tubing or Glass Tube ... https://www.researchgate.net/figure/Figure-1-A-gas-syringe-connected-by-a-rubber-tub ing-or-glass-tube-to-the-flask_fig1_313662163. Libretexts. “2.5: Reaction Rate.” Chemistry LibreTexts, Libretexts, 11 Sept. 2020, https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textboo k_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/02% 3A_Reaction_Rates/2.05%3A_Reaction_Rate. Mott, Vallerie. “Introduction to Chemistry.” Lumen, https://courses.lumenlearning.com/introchem/chapter/the-collision-theory/. Spritzler, Franziska. “10 Evidence-Based Health Benefits of Magnesium.” Healthline, Healthline Media, 3 Sept. 2018, https://www.healthline.com/nutrition/10-proven-magnesium-benefits.