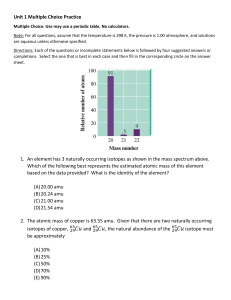
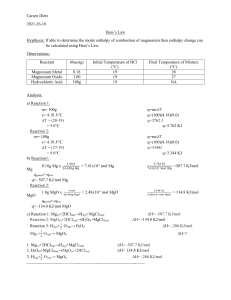
Name: ________________________ Periodic Trends Thinking Question The 1st and 2nd ionization energies (IE) of sodium and magnesium are shown in the table below. Sodium’s 1st ionization energy is clearly lower than that of magnesium. However, magnesium’s 2nd ionization energy is considerably lower than that of sodium. On this sheet, please provide an explanation for this phenomenon and submit for feedback. It may be helpful to use Bohr-Rutherford diagrams in your explanation. Na Mg 1st IE 495.8 kJ/mol 737.7 kJ/mol 2nd IE 4562.4 kJ/mol 1450.6 kJ/mol