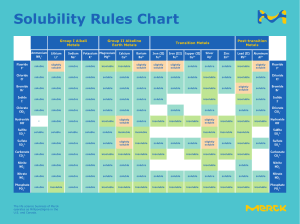
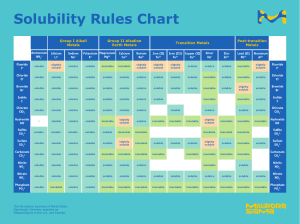
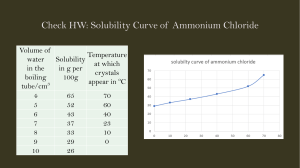
b 94 +/−1 g per 100 g The values obtained in this question and in c depend on the line of best fit. In the exam there will always be some tolerance - a range of values will be accepted. c From the graph, the solubility at 30∘ C is 10 g per 100 g of water. 40 × 10 = 4 g 100 Therefore 4 g of sodium chlorate will dissolve. d i 53 +/−1∘ C ii The solubility at 17∘ C is 7 ± 1 g per 100 g, therefore 20 − 7 = 13 g must precipitate out of the solution. Answers of 13 ± 1 g are acceptable.