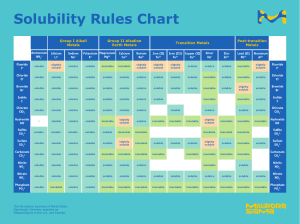
Check HW: Solubility Curve of Ammonium Chloride Volume of Temperature water Solubility at which in the in g per crystals boiling 100g appear in oC 3 tube/cm 4 65 70 5 52 60 6 43 40 7 37 23 8 33 10 9 29 0 10 26 Unit: Principles of Chemistry Topic: Solubility You are given 10 ml of water at room temperature (check room temperature using a thermometer). Starter Think, Pair, Share Conclusion: The solubility of salt In 100 g of water is 36 g Predict how many grams of salt can be dissolved in 10 ml of water. Perform an experiment to test your prediction/s. L.O. Solute Solubility (g/100 ml) Sodium chloride 36 Copper sulphate 32 Lead iodide 0.07 Lead nitrate 54 Sodium hydrogencarbonate 10 Different compounds will have differing solubilities in water. Solubility Curves ● A graph of solubility against temperature is known as a solubility curve. Solubility curves are used to find the solubility of a substance at a specific temperature. ○ ○ A line is drawn from the X-axis (temperature) up to the curve. A line is then drawn from the curve to the Yaxis (solubility). SOLUBILITY (g/100 ml) → ● The solubility of solid potassium chlorate (KClO3) in water increases as the temperature increases. TEMPERATURE (°C) → Worksheet 1 (past paper) Worksheet 2.