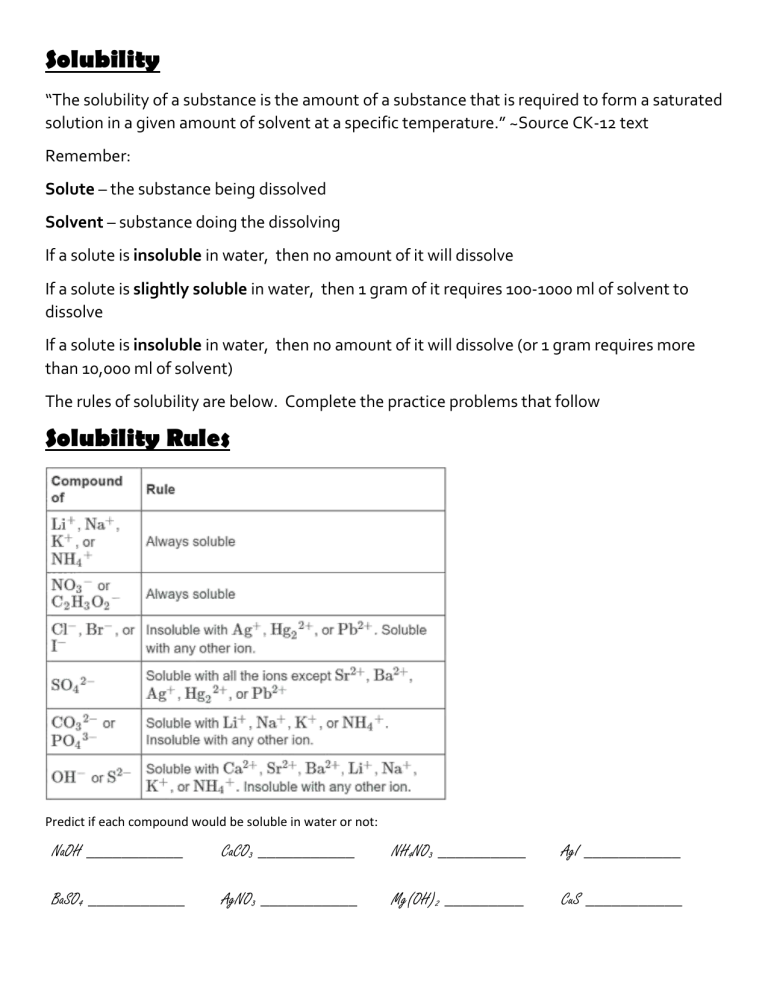
Solubility “The solubility of a substance is the amount of a substance that is required to form a saturated solution in a given amount of solvent at a specific temperature.” ~Source CK-12 text Remember: Solute – the substance being dissolved Solvent – substance doing the dissolving If a solute is insoluble in water, then no amount of it will dissolve If a solute is slightly soluble in water, then 1 gram of it requires 100-1000 ml of solvent to dissolve If a solute is insoluble in water, then no amount of it will dissolve (or 1 gram requires more than 10,000 ml of solvent) The rules of solubility are below. Complete the practice problems that follow Solubility Rules Predict if each compound would be soluble in water or not: NaOH ___________ CaCO3 ___________ NH4NO3 __________ AgI ___________ BaSO4 ___________ AgNO3 ___________ Mg(OH)2 _________ CuS ___________ Reactions and Solubility Chemistry cannot be seen directly, instead we infer what is happening from observations. Often times we know a reaction occurred because a precipitate formed. A precipitate means that one of the products of a chemical reaction was insoluble and therefore you visually see the solid it creates. One common reaction is the reaction of lead (II) nitrate and potassium iodide. Watch the quick video of the reaction and use the solubility rules to determine what you are seeing… Pb(NO3)2 (aq) + KI(aq) _________( ) + _________( ) What does the (aq) after each reactant mean? What symbol do you use if a substance is NOT soluble? For each reaction below predict the products of the reaction and then balance the equation. Determine (using the chart) if the products would be soluble (aq) or insoluble (s). 1. LiNO3 (aq) + KCl(aq) ______________( + _______________ 2. AgNO3 (aq) + KCl(aq) ______________( 3. Na2S (aq) + HCl(aq) ______________( 4. NaOH (aq) + CaBr2(aq) ______________( 5. HCl (aq) + NaOH(aq) ______________( 6. Mg(OH)2 (aq) + HCl(aq) ______________( ) + _______________ ( ) 7. Na2SO4 (aq) + SrCl2(aq) ______________( ) + _______________ ( ) ) ) ) + _______________ + _______________ ) ( ( ) ) + _______________ ) ) ( + _______________ ( ( ) ) Which of the above reactions could you do in an experiment and actually “see” product being created? Pick 1 of the reactions your identified above. How many grams of each reactant will you need to create 10 grams of the solid formed?