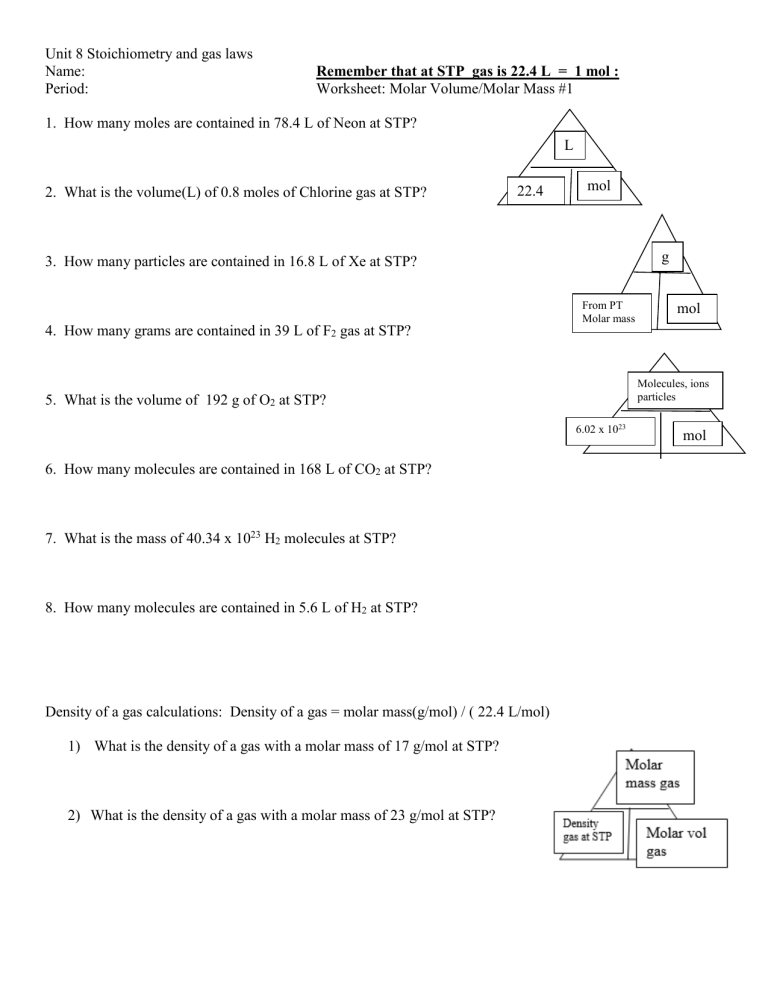
Unit 8 Stoichiometry and gas laws Name: Period: Remember that at STP gas is 22.4 L = 1 mol : Worksheet: Molar Volume/Molar Mass #1 1. How many moles are contained in 78.4 L of Neon at STP? L 2. What is the volume(L) of 0.8 moles of Chlorine gas at STP? 22.4 mol g 3. How many particles are contained in 16.8 L of Xe at STP? From PT Molar mass mol 4. How many grams are contained in 39 L of F2 gas at STP? Molecules, ions particles 5. What is the volume of 192 g of O2 at STP? 6.02 x 1023 6. How many molecules are contained in 168 L of CO2 at STP? 7. What is the mass of 40.34 x 1023 H2 molecules at STP? 8. How many molecules are contained in 5.6 L of H2 at STP? Density of a gas calculations: Density of a gas = molar mass(g/mol) / ( 22.4 L/mol) 1) What is the density of a gas with a molar mass of 17 g/mol at STP? 2) What is the density of a gas with a molar mass of 23 g/mol at STP? mol Unit 8 Stoichiometry and gas laws Solutions for when gases are not at STP 3) What is the volume of a gas that has a density of 1.96 g/L and a molar mass of 29 g/mol? 4) What is the volume of a gas that has a density of 0.96 g/L and a molar mass of 35 g/mol? 5) What is the molar mass of a gas with a density of 2.9 g/Land a molar volume of 44.8 L/mol? 6) What is the molar mass of a gas with a density of 0.5 g/L and a molar volume of 11.2 L/mol? Gas Stoichiometry and use of Ideal gas law to solve problems(solve for n(moles) using n= PV/RT first) 1. Calculate how many grams of methane (CH4) are in a sealed 800. mL flask at room temperature (22 °C) and 780. mmHg of pressure. R = 62.4 L*mmHg/mol*K 2. Dry ice is carbon dioxide in the solid state. 1.28 grams of dry ice (CO2 ) are placed into a 5.00 L evacuated chamber that is maintained at 35.1°C. What is the pressure in the chamber in atm after all the dry ice has sublimed into CO2 gas? R= 0.0821 L*atm/mol*K 3. A student conducts the following reaction in the lab at STP: 2SO2(g) + O2(g) 2 SO3(g) She reacts 5.0 L of sulfur dioxide (SO2) with excess oxygen at constant temperature and pressure. How many liters of sulfur trioxide (SO3) will she produce? (Use mole ratio step) 4. A student conducts an experiment by collecting NO2 gas at STP and uses excess oxygen: N2 + 2 O2 2NO2 How many liters of NO2 gas is produced from 3.4 L of N2 Unit 8 Stoichiometry and gas laws IB Supplemental: Gas law and stoichiometry problems 5. A student collected a sample of NO2 gas at 1.05 atm and 299 K making 45 L of NO2. How many grams of N2 were used assuming O2 was in excess? R= 0.0821 L-*atm/mol*K N2 + 2 O2 2NO2 a) Solve for moles of NO2 first using PV=nRT b) Then use stoichiometry to solve for moles of N2 used and then convert to grams using molar mass of N2 6. A student collected a sample of P2O5 gas at 0.91 atm and 287 K making 1.7 L of P2O5. How many grams of O2 was needed assuming phosphorus was in excess R= 0.0821 L-*atm/mol*K P4 + 5O2 2P2O5 c) Solve for moles of P2O5 first using PV=nRT d) Then use stoichiometry to solve for moles of O2 and convert to grams using molar mass of O2 7. A student collected a sample of NH3 gas at 303.7 kPa and 235 K making 8.7 L of NH3. How many grams of H2 were needed assuming nitrogen was in excess? R= 8.31 L-*kPa/mol*K N2 + 3H2 2NH3





