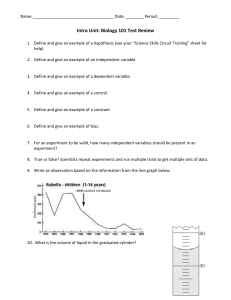
Station 1: The Universal Solvent 1. Put a pinch of salt in Beaker A, which contains 100 mL of water. 2. Stir vigorously for 1 minute and record your observations. 3. Beaker B, which contains 100 mL of oil, already has salt in it. Stir contents of Beaker B vigorously for 1 minute and record your observations. Clean-up: Leave Beaker B as you found it. Pour contents of Beaker A into the sink, rinse and dry and replace with 100 mL of fresh water. Rinse and dry stirring rods and return station as you found it. Station 2: Water on a Penny 1. Locate the penny and predict how many drops of water the penny can hold before it overflows. Record your prediction in data table. 2. Using the plastic pipette, count the number of water drops you can place on the head side of your penny before the water spills over the side. Record this number in your data table. Repeat trial. 3. Add approximately 2.5 mL of powdered laundry detergent to the beaker of tap water. Use the pipette to stir until completely dissolved. 4. Repeat Step 2 using the detergent solution. Record the numbers in your data table. Clean-up: Discard plastic pipette in the garbage. Thoroughly rinse beaker to remove all traces of detergent solution and refill with 200 mL of clean tap water. Dry penny and return station as you found it. Set Up Guide - Properties of Water ©Science Island 1 Station 3: Floating a Paper Clip 1. Obtain a paper clip, a fork and a petri dish. 2. Fill the petri dish to the top with tap water. 3. Using a steady hand, carefully use the fork to rest the paper clip onto the surface of the water. Record your observations. 4. Remove the paper clip and add a pinch of salt to the water in the petri dish. 5. Try to rest paper clip onto the surface of the water. 6. Record your observations in the data table. Clean-up: Rinse out petri dish. Also, rinse and dry off fork and paperclip. Return station as you found it. Station 4: Density Differences 1. Pour 10 mL of corn syrup into the graduated cylinder. 2. Slowly add 10 mL of colored water to the graduated cylinder by tipping it gently and pouring liquid down the side of the cylinder. 3. Slowly add 10 mL of vegetable oil to the graduated cylinder, again, by tipping the cylinder and pouring the oil down the side. 4. Carefully and slowly add 10 mL of green isopropyl alcohol to the graduated cylinder. Record your observations in your data table. 5. Securely cover the mouth of the graduated cylinder with the palm of your hand and carefully invert it 3 times. Allow the cylinder to sit for at least one minute. Record your observations. Clean-up: Pour the contents of the graduated cylinder down the sink. Wash the graduated cylinder thoroughly using dishwashing liquid and a test tube brush. Return station as you found it. Set Up Guide - Properties of Water ©Science Island 2 Station 5: “Climbing” Water 1. Obtain a strip of paper towel and using the black marker, place a small dot in the center of the strip about one inch from the bottom. Allow to dry completely. 2. Fill a 50 mL graduated cylinder with 10 mL of water. 3. Place strip of paper towel in the graduated cylinder so that the end is just touching the water and the black dot is ABOVE the water line. 4. Using the stopwatch, time how long it takes the water to reach the top. 5. Calculate the rate of water flow (cm/min) and record observations in the data table. Clean-up: Throw away the paper towel and set up the station as you found it. Station 6: Milk Rainbow 1. Obtain a paper plate and pour 50 mL of milk in to cover the bottom. 2. Add one drop of each of color of food coloring close to each other (but not touching) near the center of the plate. 3. Predict what will happen when you gently touch the milk in the middle of the plate with a clean cotton swab. 4. Touch the milk in the middle of the plate with the cotton swab. Record your observations. 5. Predict what will happen when you gently touch the milk in the middle of the plate with a cotton swab dipped in dishwashing liquid. 6. Dip the cotton swab in soap and touch it to the milk in the middle of the plate. Record your observations. Clean-up: Pour milk down the drain and throw away paper plate and used cotton swabs. Clean and set up station as you found it. Set Up Guide - Properties of Water ©Science Island 3 Station 7: Ice is Cool 1. Obtain three pieces of ice and complete Part A and Part B of this station. Part A. 1. Fill the beaker with about 30 mL warm water. 2. Add the ice cube to the beaker and immediately record the water level in the beaker. 3. Predict how much the water level will rise when the ice cube melts. 4. Complete Part B while you are waiting for ice cube to melt. 5. After the ice has melted, record your observations. Part B. 1. Squeeze two ice cubes together will all your strength for 20 seconds. 2. Let go and observe what happened to the ice cubes. 3. Go back to Part A and complete step 5. Clean-up: Pour all water down the drain and make sure your station is dry and set up as you found it. Station 1: The Universal Solvent Note: Cut and laminate each station card so they can be re-used. Set Up Guide - Properties of Water ©Science Island 4 Teacher’s Guide – Properties of Water Lab Comprehensive Material List for 7 Lab Groups Laboratory Equipment beakers 50 mL 100 mL, 4 150 mL 200 mL, 2 250 mL 500 mL, 2 graduated cylinders 100 mL 50 mL stirring rods, 2 penny paper clip plastic fork petri dish black marker, water-based stopwatch or timer 2.5 mL (½ tsp) measuring spoon test tube brush several small bowls or cups hot plate & beaker to warm water, if you don’t have access to warm tap water Optional: balance Set Up Guide - Properties of Water Consumables plastic pipettes, 7 vegetable oil, approx. 200 mL salt, approx. 45 mL (3 Tbsp) powdered laundry detergent, approx. 20 mL (4 tsp) corn syrup, 70-80 mL isopropyl alcohol, 70-80 mL paper towel, cut into 7 strips, 1.5 cm wide paper plates, 7 milk (whole works best), 400 mL food coloring (green, red, blue, yellow) cotton swabs, 14 dishwashing liquid, approx. 15 mL (1 Tbsp) + additional for cleaning ice cubes, about 2 dozen water, warm and room temperature Optional: transparent tape, small plate ©Science Island 5 Station 1: Universal Solvent Materials: 2- 200 mL beakers 100 mL water 100 mL vegetable oil salt, approx. 15 mL (1 Tbsp) in a small bowl or cup stirring rods, 2 Set Up Notes: Set out two beakers labeled A and B Add 100 mL of tap water to Beaker A Add 100 mL of vegetable oil and 3 Tbsp of salt to Beaker B (Note: Beaker B contains more salt than Beaker A so that it’s easier for students to see the salt. This is not a controlled experiment.) Optional: You may wish to add a spatula or plastic spoon for dispensing the salt. Optional: A stopwatch or timer could be used for timing. Procedure: 1. Put a pinch of salt in Beaker A, which contains 100 mL of water. 2. Stir vigorously for 1 minute and record your observations. 3. Beaker B, which contains 100 mL of oil, already has salt in it. Stir contents of Beaker B vigorously for 1 minute and record your observations. Clean-up: Leave Beaker B as you found it. Pour contents of Beaker A into the sink, rinse and dry and replace with 100 mL of fresh water. Rinse and dry stirring rods and return station as you found it. Set Up Guide - Properties of Water ©Science Island 6 Station 2: Water on a Penny Materials: penny plastic pipettes, 7 powdered laundry detergent, approx. 20 mL (4 tsp) in a small bowl or cup 250 mL beaker filled with about 200 mL of water 2.5 mL measuring spoon (or ½ tsp) Set Up Notes: Optional: You may prefer to give students the opportunity to use a balance to measure the mass of the laundry detergent. If so, each group will need approx. 3 grams of detergent, for a total of 21 grams. If you choose this option, please remember to change the station instruction card accordingly. Procedure: 1. Locate the penny and predict how many drops of water the penny can hold before it overflows. Record your prediction in the data table. 2. Using the plastic pipette, count the number of water drops you can place on the head side of your penny before the water spills over the side. Record this number in your data table. 3. Perform an additional trial and record the number of drops in your data table. 4. Add approximately ½ teaspoon of powdered laundry detergent to the beaker of water. Use the pipette to stir until the detergent is completely dissolved. 5. Repeat Step 2 using the detergent solution. Record the number in your data table. 6. Perform an additional trial. Clean-up: Discard plastic pipette in the garbage. Thoroughly rinse beaker to remove all traces of detergent solution and refill with 200 mL of clean tap water. Dry penny and return station as you found it. Set Up Guide - Properties of Water ©Science Island 7 Station 3: Floating a Paper Clip Materials: paper clip plastic fork petri dish salt, approx. 20 mL (4 tsp) in a small bowl or cup water, about 300 mL in a 500 mL beaker Optional: small dish or plate, such as styrofoam Set Up Notes: Having the water available at the station in a large beaker or flask will make it easier for students to fill the petri dish to the top. You may wish to place the petri dish on a small dish or plate to catch water that may be spilled during filling or clean-up. If students have trouble using the fork in step 3, they may try slowly sliding the paper clip horizontally from the side of the petri dish onto the surface of the water. Procedure: 1. Obtain a paper clip, a fork and a petri dish. 2. Fill the petri dish to the top with tap water. 3. Using a steady hand, carefully use the fork to rest the paper clip onto the surface of the water. Record your observations. 4. Remove the paper clip and add a pinch of salt to the water in the petri dish. 5. Try to rest paper clip onto the surface of the water. 6. Record your observations in the data table. Clean-up: Rinse out petri dish. Also, rinse and dry off fork and paperclip. Return station as you found it. Set Up Guide - Properties of Water ©Science Island 8 Station 4: Density Differences Materials: 100 mL beakers, 4, labeled and filled with 70-80 mL of the following liquids: -corn syrup -colored water, red or blue -vegetable oil -isopropyl alcohol with green food coloring added 100 mL graduated cylinder dishwashing liquid and a test tube brush for clean-up food coloring, as listed above Set Up Notes: Any color of food coloring may be used for the water and alcohol, but choose colors that contrast well with each other for a better effect. Food coloring may also be added to corn syrup if you prefer. If you are interested in adding more layers, honey is more dense than corn syrup, dishwashing liquid has a density between that of corn syrup and water, and baby oil is less dense than isopropyl alcohol. After using the food coloring, leave it for students at station 6. Procedure: 1. Pour 10 mL of corn syrup into a 100 mL graduated cylinder. 2. Slowly add 10 mL of colored water to the graduated cylinder by tipping it gently and pouring liquid down the side of the cylinder. 3. Slowly add 10 mL of vegetable oil to the graduated cylinder by again by tipping and pouring down side. 4. Carefully and slowly add 10 mL of green isopropyl alcohol to the graduated cylinder. 5. Record your observations in your data table. 6. Securely cover the mouth of the graduated cylinder with the palm of your hand and carefully invert it 3 times. Allow the cylinder to sit for at least one minute. Record your observations. 7. Clean-up: Pour the contents of the graduated cylinder down the sink and wash the cylinder thoroughly using dishwashing liquid and a test tube brush. Return station as you found it. Set Up Guide - Properties of Water ©Science Island 9 Station 5: “Climbing” Water Materials: paper towel – cut into 7 strips that are about 1.5 cm wide black marker (vis-à-vis works well, or some other water based) 50 mL graduated cylinder water, about 100 mL in a 150 mL beaker stopwatch Optional: transparent tape Set Up Notes: Paper towels vary in size, so just make sure your strips are long enough to touch the water in the bottom of the graduated cylinder. Procedure: 1. Obtain a strip of paper towel and, using the black marker, place a small dot about one inch from the bottom. Allow to dry completely. 2. Add 10 mL of water to the 50 mL graduated cylinder. 3. Place the strip of paper towel in the graduated cylinder so that the end is just touching the water and the black dot is ABOVE the water line. You may want to use tape to secure the paper strip at the top of the graduated cylinder. 5. Using the stopwatch, time how long it takes the water to reach the top. 6. Calculate the rate of water flow (cm/min) and record observations in the data table. 7. Clean-up: Throw away the paper towel and set up the station as you found it. Set Up Guide - Properties of Water ©Science Island 10 Station 6: Milk Rainbow Materials: paper plates, 7 milk (2% or whole), about 400 mL in a 500 mL beaker food coloring - blue, red, green, yellow cotton swabs, 14 dishwashing liquid, approx. 15 mL (1 Tbsp) in a small cup or bowl Set up Notes: If you think students will make a mess with the milk in the large beaker, you may decide to set up small beakers or graduated cylinders with 50 mL of milk ????? Procedure: 1. Obtain a paper plate and pour 50 mL of milk in to cover the bottom. Allow the milk to settle. 2. Add one drop of each of color of food coloring close to each other (but not touching) near the center of the plate. 3. Predict what will happen when you gently touch the milk in the middle of the plate with a clean cotton swab. 4. Touch the milk in the middle of the plate with the cotton swab and record your observations. 5. Predict what will happen when you gently touch the milk in the middle of the plate with a cotton swab dipped in dish soap. 6. Dip the cotton swab in soap and touch the milk in the middle of the plate. Record your observations. 7. Clean-up: Pour milk down the drain and throw away paper plate and used cotton swabs. Clean and set up station as you found it. Set Up Guide - Properties of Water ©Science Island 11 Station 7: Ice is Cool Materials: ice cubes, 21 beaker, 50 mL warm water, about 250 mL optional: stopwatch or timer Set Up Notes: Warm tap water is all that is required, but if you don’t have warm water running to your classroom, you may need to set up a hot plate to warm water in a large beaker or flask; about 100 - 110 degrees is all you need. In a pinch, you can use room temperature water, but it will, of course, take longer for the ice cube to melt. I like to do this experiment in a graduated cylinder, because it is easier to see the water volume. But, it is sometimes difficult to find ice cubes that are skinny enough to fit into the cylinder. You can also use a small transparent cup and students can mark the water level on the outside. Procedure: Obtain three pieces of ice and complete Part A and Part B of this station. Part A. 1. Fill the beaker with about 30 mL warm water. 2. Add the ice cube to the beaker and immediately record the water level in the beaker. 3. Predict how much the water level will rise when the ice cube melts. 4. Complete Part B while you are waiting for ice cube to melt. 5. After the ice has melted, record your observations. Part B. 1. Squeeze two ice cubes together will all your strength for about 20 seconds. 2. Let go and observe what happened to the ice cubes. Record your observations. 3. Go back to Part A and complete step 5. Clean-up: Pour all water down the drain and make sure your station is dry and set up as you found it. Set Up Guide - Properties of Water ©Science Island 12