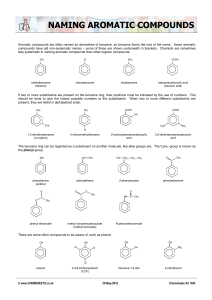
IBSL/HL Chem Name: _____________________________ 10.1.D Organic Nomenclature 1. For each of the following molecules give its class and its IUPAC name: a. CH3CH2CH2COOH b. CHCl2CH2CH3 c. CH3CH2COCH3 d. CH3COOCH3 e. CH3CH2OCH3 f. CH3CH2CH2CH2COOCH2CH3 2. Give the structural formulas of the following molecules (condensed or full): a. Hexanoic acid b. Butanal c. Pent-1-ene d. 1-bromo-2-methylbutane e. Ethyl methanoate f. Methoxypropane g. But-2-yne 3. Which of the following is an amine? a. CH3CH2NH2 b. CH3CONH2 c. CH3CH2CN d. C2H5CONH(CH3) 4. Which compound is a member of the same homologous series as 1-bromopropane? a. 1-iodopropane b. 1,2-dibromopropane c. 1-promopropene d. 1-bromopentane 5. Draw and name all structural isomers of C3H3Cl5. 6. Which formula is that of a secondary halogenoalkane? a. CH3CH2CH2CH2Br b. CH3CHBrCH2CH3 c. (CH3)2CHCH2Br d. (CH3)3CBr 7. Describe the bonding in a benzene molecule and use it to explain benzene’s stability. Fisk 2019 1