General Chemistry 2 Online Module: Kinetic Molecular Model
advertisement

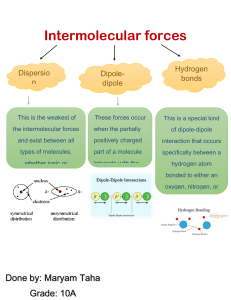
A.Y. 2020-2021 2 CHEMISTRY Senior High School Online Class Module Prepared by: The Science Faculty Capitol University- Senior High School Grade: 12 Subject: General Chemistry 2 Subject Description: This subject is designed to enhance the understanding of the composition, structure, and properties of matter; quantitative principles, kinetics, and energetics of transformations of matter; and fundamental concepts of chemistry Textbook: DIWA Senior High School Series: General Chemistry 2 (DIWA Learning Systems, Inc.) INTRODUCTION: Good day! Welcome to General Chemistry 2 Online Class Module. This module was specifically designed for you to allow recall and enhance mastery of the main content regarding fundamental concepts of chemistry, paralleled to the mandated set of topics by the Department of Education through their curriculum guides. This module not only will redirect you to the different subject matter, it also provides meaningful assessment tools and opportunities for interactive discussions (in which in this case, through set online synchronous classes) to allow holistic learning opportunities. This module is designed in such a way you, the learners, can go through the topics in your most convenient time and pace. Make sure to read some of the reminders below critically, and for further questions, please don’t hesitate to contact your instructor: Below are set of topics that will be main references for you to answer your activity sheets, accordingly to the set schedules. The references (textbook pages, web article and multimedia files, etc.) are also indicated in each part of the module. The activity sheets/worksheets will be provided to you via softcopy by your instructor in “.docx” format for you to edit and answer. Take your time in reading the content of the subject matter. Take note of the highlighted terminologies/formula/notes in each topic. Take note of some points that might not be clear to you so you can raise these concerns to your instructor through synchronous sessions. You will be receiving constant updates and monitoring from your instructor to make sure you are able to comply with the said activity sheets. Make sure to comply with all the set activities and worksheets indicated for each topic and submit these on the set deadlines Module: Week 1 “Kinetic Molecular Model of Liquids and Solids” TARGET COMPETENCY The learners demonstrate and understanding of… The properties of liquids and solids to the nature of forces between particles Phase changes in terms of the accompanying changes in energy and forces between particles TASK Read and analyze all the contents of this module. Make sure to open/watch the attached instructional files, articles, videos, etc. (if there are any). These will help you further understand the topics under discussion. Answer the following tests/quizzes (if there are any) and make sure to submit them on time. IMPORTANT TERMINOLOGY/ FORMULA Kinetic Malleability Ductility Freezing Melting Vaporization Sublimation Intermolecular forces of attraction London-dispersion force Polarization Temporary dipoles Electronegativity Dipole-dipole interaction Ion-dipole interaction Anion Cation Hydrogen bond Viscosity Surface tension Surface free energy Vapor pressure Standard heat of vaporization Condensation Equilibrium Vapor pressure of a liquid Volatility Distillation Boiling point Acid Base Autoprotolysis Leveling effect of water Buffer LESSON PROPER THE KINETIC MOLECULAR MODEL “KINETIC”- comes from the Greek word “kinein” which means “to move” The KINETIC MOLECULAR MODEL is used to explain the properties of liquid and solid molecules, as well as the forces of attraction that account for such properties. The KINETIC ENERGY of the particle is the energy needed to keep the particles moving, which is dependent upon temperature. The particles of each phase of matter are explained by the following postulates: The particles of solids are closely packed together. Because of their compact arrangement, solids have definite sizes and shapes. This particle arrangement is also responsible for their malleability (the ability of a solid to be formed into thin sheets without breaking) and ductility (the ability of the solid to be formed into thin wires). Different states of solids may exist as solid particles can rearrange differently and follow a specific stacking pattern depending on the current temperature and pressure conditions when the solid is being formed. Solids usually have strong forces of attraction. The particles of liquids are slightly far from one another. This amount of space enables liquids to flow and take the shape of their containers. Thus, they have no definite sizes and shapes The particles of gases move freely and are very far from one another, which is why you cannot see them. Furthermore, gases diffuse easily. Intermolecular Forces of Attraction (IMFA) This pertains to forces that hold individual particles such as atoms, molecules, or ions together. The strength of the IMFA is dependent on the following: arrangement of the particles the proximity of particles relative to one another the nature of the interacting particles The Intermolecular forces of attraction influence the resulting properties of solids, liquids, and gases, such as boiling point, viscosity, vapor pressure, and heat of vaporization. Generally, the stronger the IMFA, the greater is the required amount of energy to overcome these forces The Intramolecular forces of attraction are responsible for interactions within a molecule, such as covalent and ionic bonds. These are the several types of IMFA, also called as van der Waals forces (named after the Dutch scientist Johannes Diderik van der Waals, 1837-1923) 1. London dispersion force (named after the German-born physicist Fritz London) - is the weakest among the intermolecular forces. This is caused by polarization (the distortion of the electron cloud brought about by the presence of a highly charged particle, in this case, the electron cloud of one atom is attracted to the positively charged nucleus of another atom. The number of electrons in an atom affects the polarizability of the atom. As a result, the larger the electron cloud, the higher the chances of the atom getting attracted by a positively charged particle. This results in the formation of temporary dipoles (one end of an atom is partially positive and the other end is partially negative. To summarize, the London dispersion force happens when one molecule with a temporary dipole exerts a weak attractive force on another molecule. And since it is caused by polarization, the strength of this force depends on the number of electrons present. (Ex: CO2, H2, O2, and N2, halogens such as Xe and Ar). Because of the relatively weak attraction of this type of IMF, it is significant over a short distance only, which is approximately 3 x 10-9 meters. However, the London dispersion force is responsible for the condensation and solidification of these molecules. 2. Dipole-dipole interaction - it occurs between partially positive (δ+) and partially negative (δ-) ends. This interaction is observed in polar covalent molecules such as amino acids wherein the electrons are shared both by oxygen and carbon atoms. However, due to the electronegativity of the oxygen atom, it assumes a partially negative charge, whereas the carbon atom assumes a partially positive charge. This type of force is effective over a short distance only, as it is weak, containing only 1% of the strength of the ionic bonds. Moreover, an increase in temperature diminishes the strength of a dipole-dipole interaction, which may explain the observed volatility of certain polar covalent compounds such as sulfur dioxide. 3. Ion-dipole interaction – this arises from the interaction between an ion and a polar molecule. If the molecule is an anion, it will be attracted to the partially positive end of the polar molecule; however, if the molecule is a cation, it will be attracted to the partially negative end of the polar molecule. The ion-dipole interaction is responsible for the formation of cations in a solution. Cations are formed from ionic solids such as CaCl2. Elements belonging to groups I and II of the periodic table, such as calcium (Ca2+), lose electrons easily, and thus form cations. 4. Hydrogen bond – it is a special kind of a dipole-dipole interaction which is formed when hydrogen bonds with fluorine, oxygen and nitrogen. In a hydrogen bond, the partially positive end of the hydrogen atom is attracted to the partially negative end of fluorine, oxygen, or nitrogen. Generally, hydrogen bonds are still weaker than ionic or covalent bonds, but they are the strongest IMFA (when the hydrogen bond is present between two atoms of two different molecules). The strength of the hydrogen bond is the reason for the high melting and boiling points of water, ammonia, and alcohols such as methanol. A flow diagram for identifying the IMFA -END-