Construction Materials for ABE: Metals, Wood, Ceramics, Polymers
advertisement

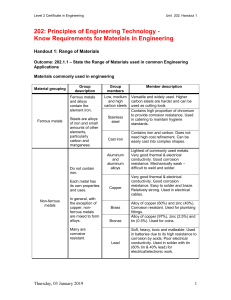
LABO CAMPUS - COLLEGE OF AGRICULTURE AND NATURAL RESOURCES LESSON 2 ON MATERIALS AND PROCESS FOR ABE (AG-Eng1) for BSABE 2A & 2B B. Construction Materials B.1. Metals- This type of materials has characteristics like, high electrical and thermal conductivity, the ability to be deformed or cut into new shapes without breaking, and high mechanical strength. Since metals must be reduced from chemical compounds, they tend to be somewhat more costly than non-metallic materials, and they are often vulnerable to corrosion damage as the metals react with their environment to re-form those compounds. They tend to be shiny and malleable. Metals have these characteristics because they have nonlocalized electrons. Steel is a metal alloy of iron and carbon and often other alloying material in its composition to make it stronger and more fracture-resistant than iron. Stainless steels resist corrosion and oxidation because of the additional chromium in their make-up. Because it is so strong compared to its weight and size, structural engineers use it for the structural framework of tall modern buildings and large industrial facilities. Some of its qualities include: • • • • • Steel has high strength-to-weight and strength-to-size ratios. It’s high-cost relative to other metals. Structural engineers can consult on choosing the most costeffective sizes to use in a house to support the actual load on the building. Steel is less time-consuming to install than concrete. It can be installed in any environment. Steel can be susceptible to corrosion if improperly installed or maintained Chrome, gold, and silver are generally used for finishes or decoration because they lack the tensile strength of steel. B.2. Wood - Among the oldest, or perhaps the oldest, of building materials, wood has been used for thousands of years and has properties that make it an ideal building material—even in the days of engineered and synthetic materials. For construction use, wood pieces are machine-planed and cut into standard dimensions, such as 2”x4” (1.5”x3.5” actual) and 2”x6,” (1.5”x5.5” actual) so that their measurements can be accurately factored into building plans—this is known as dimensional lumber. Wood in larger sizes is usually referred to as timber or beams and is often used to construct the frames of large structures like bridges and multi-story buildings. Some tree species are better for some uses and for use in some climates than others. Structural engineers and architects can determine which type of wood is ideal for a construction project. • It is readily available and an economical natural resource. • • • • Wood is relatively lightweight and easy to standardize in size. It provides good insulation, which is why many architects and engineers like using it for homes and residential buildings. Wood has high tensile strength—keeping its strength while bending—and is very strong when being compressed vertically. Because it is lightweight and needs to be pressure treated to come into contact with surrounding soil, wood is a less popular choice for foundations or basement walls. (However, permanent wood foundations, known as PWFs, are gaining traction among builders thanks to the warm and inviting wood basement living space they offer.) More often, wood-framed homes usually have a reinforced concrete or pier and beam foundations. are generally compounds between metallic and nonmetallic elements and include such compounds as oxides, nitrides, and carbides. Typically they are insulating (not electrical or thermally conductive) and resistant to high temperatures and harsh environments (corrosion resistant). They usually have lower electrical and thermal conductivity, higher stiffness, good resistance to corrosive environments, and lower fracture toughness than metals. With the exception of glasses, ceramics usually cannot be reshaped easily. To shape a ceramic, a mixture of ceramic powders, water, and binder materials is molded into the desired dimensions to form a temporary shape. These temporary shapes called "green bodies" are then dried to remove water and heated to allow the binder materials to oxidize, leaving the ceramic powder particles to bond to each other during the high temperature baking. Ceramic engineering is the science and technology of creating objects from inorganic, non-metallic materials. This is done either by the action of heat, or at lower temperatures using precipitation reactions from high-purity chemical solutions. B.3. Ceramics- B.4. Polymers-Plastics (or polymers) are generally organic compounds based upon carbon and hydrogen. They are very large molecular structures. Usually they are low density and are not stable at high temperatures. They can be readily formed into complex shapes. Their strength, stiffness, and melting temperatures are generally much lower than those of metals and ceramics. Their light weight, low cost, and ease of forming make them the preferred material for many engineering applications. B.5. Composites - a combination of two or more materials differing in form or composition. The differnet parts still have the same features they originally did, that is, they do not dissolve or merge completely into one another, however, their properties are enhanced by each other. Normally, the components can be physically identified and exhibit an interface (boundary) between one another. Fiberglass, a combination of glass and a polymer, is an example. Concrete and plywood are other familiar composites. Many new combinations include ceramic fibers in metal or polymer matrix. Corrosion is the destructive attack of a material by reaction with its environment. The serious consequences of the corrosion process have become a problem of worldwide significance. In addition to our everyday encounters with this form of degradation, corrosion causes plant shutdowns, waste of valuable resources, loss or contamination of product, reduction in efficiency, costly maintenance, and expensive overdesign. It can also jeopardize safety and inhibit technological progress. Step 1 iron + oxygen --> iron oxide Step 2 iron oxide + water --> hydrated iron oxide (rust) Corrosion is the atmospheric oxidation of metals. That means that oxygen combines with the metal and forms a new layer. This layer can be good or bad. By far the most important form of corrosion is the rusting of iron and steel. Rusting is a process of oxidation in which iron combines with water and oxygen to form rust, the reddish-brown crust that forms on the surface of the iron. Because iron is so widely used, e.g., in building construction and in tools, its protection against rusting is important. Rusting can be prevented by excluding air and water from the iron surface, e.g., by painting, oiling, or greasing, or by plating the iron with a protective coating of another metal. Many alloys of iron are resistant to corrosion. Stainless steels are alloys of iron with such metals as chromium and nickel; they do not corrode because the added metals help form a hard, adherent oxide coating that resists further attack. Although metals like aluminum, chromium, and zinc corrode more readily than iron, their oxides form a coating that protects the metal from further attack. Rust is brittle and flakes off the surface of the iron, continually exposing a fresh surface. Thus these metals might be a better selection choice for a product that will be exposed to rusting conditions, like water and air. Recognizing the symptoms and mechanism of a corrosion problem is an important preliminary step on the road to finding a convenient solution. There are basically five methods of corrosion control: • Change to a more suitable material • • Modifications to the environment with the use of inhibitors • • Use of protective metallic or organic coatings • • Design modifications to the system or component • o o Provide adequate ventilation and drainage to minimize the accumulation of condensation o o Avoid depressed areas where drainage is inadequate o o Avoid the use of absorptive materials (such as felt, asbestos and fabrics) in contact with metallic surfaces) o o Prepare surfaces adequately prior to the application of any protective coating system. o o Provide easy access for the purposes of corrosion inspection and maintenance work • Information care of Corrosion-doctors for more information about: Specific Corrosion of Metals, Theory of Corrosion, or a Corrosion Glossary click on link Choosing the right process It is all very well to choose the perfect material, but somehow we have to make something useful out of it! An important part of understanding a product is to consider how it was made - in other words what manufacturing processes were used and why. There are 2 important stages to selecting a suitable process: Technical performance: can we make this product with the material and can we make it well? Economics: if we can make it, can we make it cheaply enough? To find out about the different processing of materials click on the following button, click on the process you want to find out about on the left, and then read about it on the right, make sure you also click on the four icons (Overview, Materials and Shapes, Economics, and Typical Products) above each description : Conductivity is the measure of the ease at which an electric charge or heat can pass through a material. A conductor is a material which gives very little resistance to the flow of an electric current or thermal energy. Materials are classified as metals, semiconductors, and insulators. Metals are the most conductive and insulators (ceramics, wood, plastics) the least conductive. Electrical conductivity tells us how well a material will allow electricity to travel through it. Many people think of copper wires as something that has great electrical conductivity. Thermal conductivity tells us the ease upon which thermal energy (heat for most purposes) can move through a material. Some materials like metals allow heat to travel through them quite quickly. Imagine that with one hand you are touching a piece of metal and with the other, a piece of wood. Which material would feel colder? If you said, "metal," you would be correct. But, in fact, both materials are in fact the same temperature. This is relative thermal conductivity. Metal has a higher heat transferability, or thermal conductivity, than wood, letting the heat from your hand leave faster. If you want to keep something cold the best idea is to wrap it in something that does not have a high heat transferability, or high thermal conductivity, this would be an insulator. Ceramics, and polymers are usually good insulators, but you have to remember that polymers usually have a very low melting temperature. That means if you are designing something that will get very hot the polymer might melt, depending on its melting temperature. Electrical and thermal conductivity are closely related. For the most part good electrical conductors are also good thermal conductors. Many products will contain both conductors and insulators- the conductors take the electricity or thermal energy where it is wanted and the insulators prevent it from getting where it isn't wanted. Silver has the highest electrical conductivity of all metals. In fact, silver defines conductivity - all other metals are compared against it. On a scale of 0 to 100, silver ranks 100, with copper at 97 and gold at 76. Because of this property, and because it doesn't spark easily, silver is commonly used in electrical circuits and contacts. Silver is also utilized in batteries where dependability is mandatory and weight restrictions apply, such as those for portable surgical tools, hearing aids, pacemakers and space travel. LINKS http://www.physics4kids.com/files/elec_conduct.html Lesson plan for teachers about conductivity- http://www.infinitepower.org/pdf/09-LessonPlan.pdf Force is a push or a pull. Leaves and apples fall to the ground because the force of gravity pulls them. When boys and girls swing, sometimes a friend pushes them to help them get started. This push is a force. When an airplane lands on a runway, the force of gravity pulls it down toward the ground. When you open or close a door, you are exerting a force on the door. Check out these really fun tutorials from Building Big to get an idea of they types of forces and how they affect different materials. Now think about your product and how you're redesigning it, think about the forces it is undergoing, are they compression, tension, etc. and what materials work best for those kind of forces. A hint is to think of the strength and the stiffness of the original material, and make sure that you are at least as strong and stiff as that material. https://www.lehigh.edu/~amb4/wbi/kwardlow/WBIevidence.htm Assignment: 1. Give example of each construction materials: a. Metals b. Woods c. Ceramics d. Polymers e. Composites 2. Make Drawing or Pictures of each and discuss. Prepared by: DAVE Y. RIEZA, RPABE Instructor