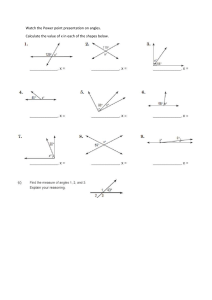
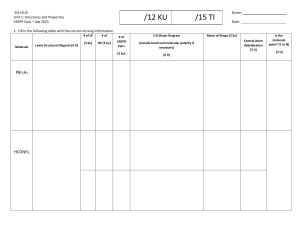
VSEPR Introductory Lab Activity Procedure: 1) Draw the Lewis structure for each molecule. 2) Use the model kit to make a 3-D model of the structure using the correct number of atoms of each element. Black = carbon Blue = nitrogen Green = halogens Red = oxygen White = hydrogen 3) Sketch an image of the 3-D model for each compound. Use crayons to indicate the types of atoms. Make sure to illustrate in your drawing whether you used a single, double bond, or triple bond. Formula HI OCl2 CH4 NH3 O2 Lewis dot structure 3-D drawing (include measure of bond angles on some of them) VSEPR shape CCl4 CH2O NCl3 CH3Cl N2 CO2 HCN H2O Summary: Put the molecules into categories according to who has similar shapes. Use the following criteria to aid you: How many atoms are around the central atom? Are the atoms all in the same plane or is the molecule 3d? What are the bond angles? Under each category, list the characteristics that each of those molecules has in common with the others in the same category. The names of the VSEPR shapes are linear, tetrahedral, trigonal planar, trigonal pyramidal, and bent. Try to match up those shapes with the categories you came up with. Compare and contrast the following pairs (include comparisons between Lewis structures and 3D shapes) – include similarities and differences: H2O and CO2 NCl3 and CH2O Teacher’s Guide This is an introduction to VSEPR. Students should have prior knowledge of how to draw Lewis structures. I use this before a lesson on molecular geometry and VSEPR. Students draw the Lewis structures, then make the shapes with the ball and stick models and draw the shapes from the models. If you want them to use a protractor to measure bond angles on a few, that helps them recognize where those numbers come from when you teach that. I have them leave the VSEPR column blank during the lab activity. They then go through the questions with their group to notice the patterns – how the Lewis structures predict the 3-d shape. Students really want to focus on single versus double versus triple bonds and I have to remind them not to – to focus on the arrangements of the ATOMS around each other. The lesson on VSEPR is based off of the summary questions at the end – we look at the 3D model, the Lewis structure, and discuss it together. Then, after I’ve taught the VSEPR names, they fill in the last column as a review. blog here here click here to go shop here here