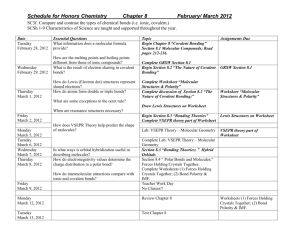
MYP 10 Chemistry 2013-14 Bonding Worksheet
advertisement
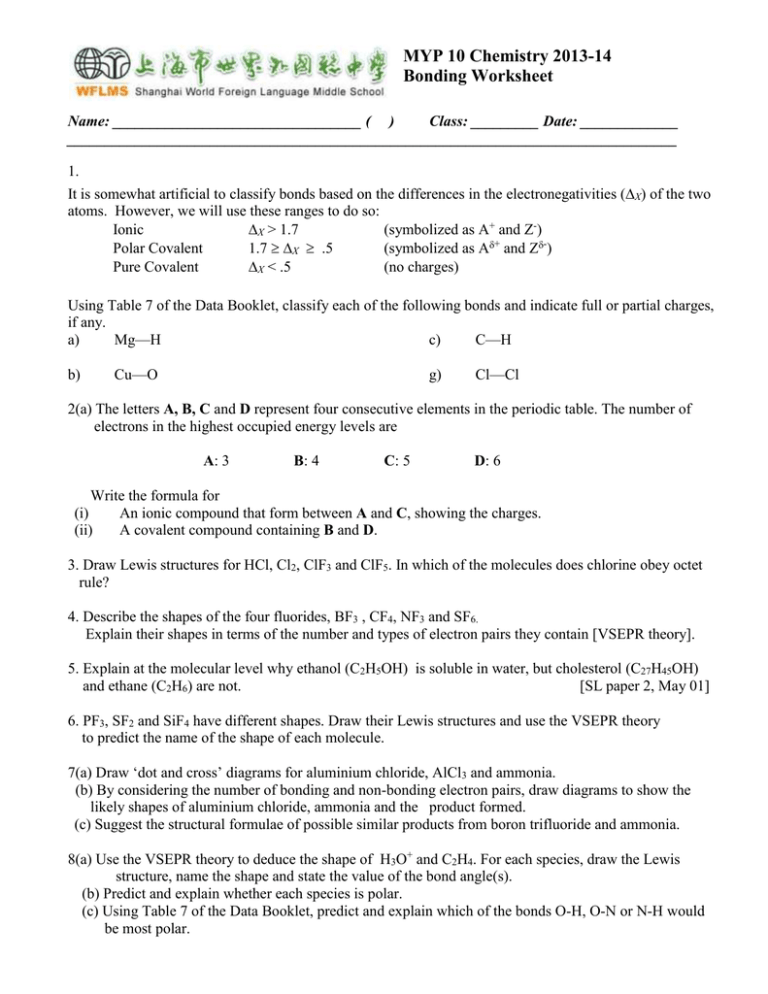
MYP 10 Chemistry 2013-14 Bonding Worksheet Name: _________________________________ ( ) Class: _________ Date: _____________ _________________________________________________________________________________ 1. It is somewhat artificial to classify bonds based on the differences in the electronegativities (X) of the two atoms. However, we will use these ranges to do so: Ionic X > 1.7 (symbolized as A+ and Z-) Polar Covalent 1.7 X .5 (symbolized as A+ and Z-) Pure Covalent X < .5 (no charges) Using Table 7 of the Data Booklet, classify each of the following bonds and indicate full or partial charges, if any. a) Mg—H c) C—H b) Cu—O g) Cl—Cl 2(a) The letters A, B, C and D represent four consecutive elements in the periodic table. The number of electrons in the highest occupied energy levels are A: 3 B: 4 C: 5 D: 6 Write the formula for (i) An ionic compound that form between A and C, showing the charges. (ii) A covalent compound containing B and D. 3. Draw Lewis structures for HCl, Cl2, ClF3 and ClF5. In which of the molecules does chlorine obey octet rule? 4. Describe the shapes of the four fluorides, BF3 , CF4, NF3 and SF6. Explain their shapes in terms of the number and types of electron pairs they contain [VSEPR theory]. 5. Explain at the molecular level why ethanol (C2H5OH) is soluble in water, but cholesterol (C27H45OH) and ethane (C2H6) are not. [SL paper 2, May 01] 6. PF3, SF2 and SiF4 have different shapes. Draw their Lewis structures and use the VSEPR theory to predict the name of the shape of each molecule. 7(a) Draw ‘dot and cross’ diagrams for aluminium chloride, AlCl3 and ammonia. (b) By considering the number of bonding and non-bonding electron pairs, draw diagrams to show the likely shapes of aluminium chloride, ammonia and the product formed. (c) Suggest the structural formulae of possible similar products from boron trifluoride and ammonia. 8(a) Use the VSEPR theory to deduce the shape of H3O+ and C2H4. For each species, draw the Lewis structure, name the shape and state the value of the bond angle(s). (b) Predict and explain whether each species is polar. (c) Using Table 7 of the Data Booklet, predict and explain which of the bonds O-H, O-N or N-H would be most polar. *9. By reference to the structure and bonding in NaCl and SiCl4: (i) State and explain the differences in electrical conductivity in the liquid state. (ii) Predict an approximate pH value for the solutions formed by adding each compound separately to water. Explain your answer.