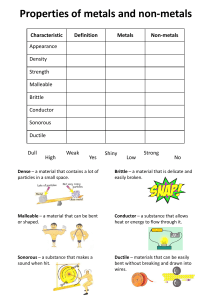
DUCTILE AND BRITTLE MATERIALS The two terms ductile and brittle are used to describe two physical changes in substances. Ductile substances can be easily hammered or stretched into thin wires without breakage. This physical property of a material that is associated with the ability to be hammered thin or stretched into wire without breaking is explained as ductility. The materials having this property are known as ductile materials. A ductile material can easily be drawn into wires. Metals are the best examples of ductile materials. For example, gold, silver, copper are ductile. Although aluminum is a metal, it is not ductile. The ductility of metals can be high or low. Copper is highly ductile and can be drawn into thin wires without breakage. Moreover, the temperature also has a great influence on the ductility of metals. Generally, when metals are heated, they become less brittle. Some nonmetals also become ductile when heated, so they can be stretched. But lead (Pb) is an exception. It becomes more brittle when heated. Ductility in metals for example is due to the high degree of metallic bonds present in metals. Metals have a lattice structure where valence electrons are delocalized from the metal atoms, and the electrons are shared between atoms forming metallic bonds. Due to this electron delocalization, metal atoms can slide past each other, allowing it to be drawn into thin wires. Major examples for ductile materials are metals such as; gold, silver, copper, erbium, terbium, and samarium. Examples of metals that are not very ductile include tungsten and high-carbon steel. Nonmetals are not generally ductile. Brittle substances are liable to break easily. These substances are hard, and cannot be hammered or stretch like ductile substances; instead, they break. The term brittle describes materials that are easily broken, cracked, or snapped. Materials break when a stress is applied to them. Brittle materials break without any deformation. Therefore, they cannot be stretched like ductile substances. The breaking of brittle substances objects with a snapping sound. When these objects are broken, the edges fit each other because there is no deformation before breakage. Many materials such as ceramic and glass are brittle. Even steel become brittle at low temperatures. When a stress is applied to a material, there is a limit to which the stress can be tolerated by that material. When that limit is reached, the material may either deform or break down. Ductile materials deform at this point while brittle materials break apart. Examples of brittle materials include ceramic and glass Brittleness in different materials a. Polymers Mechanical characteristics of polymers can be sensitive to temperature changes near room temperatures. For example, poly(methyl methacrylate) is extremely brittle at temperature 4˚C,[1] but experiences increased ductility with increased temperature. Amorphous polymers are polymers that can behave differently at different temperatures. They may behave like a glass at low temperatures (the glassy region), a rubbery solid at intermediate temperatures (the leathery or glass transition region), and a viscous liquid at higher temperatures (the rubbery flow and viscous flow region). This behavior is known as viscoelastic behavior. In the glassy region, the amorphous polymer will be rigid and brittle. With increasing temperature, the polymer will become less brittle. b. Ceramics Ceramics are generally brittle due to the difficulty of dislocation motion, or slip. There are few slip systems in crystalline ceramics that a dislocation is able to move along, which makes deformation difficult and makes the ceramic more brittle. Ceramic materials generally exhibit ionic bonding. Because of the ions’ electric charge and their repulsion of like-charged ions, slip is further restricted. c. Metals Some metals exhibit brittle characteristics due to their slip systems. The more slip systems a metal has, the less brittle it is, because plastic deformation can occur along many of these slip systems. Conversely, with fewer slip systems, less plastic deformation can occur, and the metal will be more brittle. For example, HCP (hexagonal close packed) metals have few active slip systems, and are typically brittle. Differences Between Ductile and Brittle A. By Definition: Ductile materials can be drawn into wires by stretching. Brittle: Brittle materials break, crack or snap easily. B. By Deformation: Ductile materials show deformation. Brittle materials do not show deformation. C. By Factors Affecting the Process Ductility is affected by temperature. Brittleness is affected by pressure (or stress). Conclusion Materials can be named as ductile materials or brittle materials based on their response to an applied stress on them. The main difference between ductile and brittle materials is that ductile materials are able to be drawn out into thin wires whereas brittle materials are hard but liable to break easily