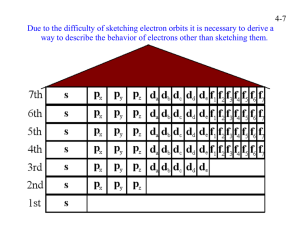
SHS General Chemistry 1 Quarter 2 – Week 1 Module 1- Quantum Numbers General Chemistry 1 Grade 11/12 Quarter 2 - Module 1- Quantum Numbers First Edition, 2020 Copyright © 2020 La Union Schools Division Region I All rights reserved. No part of this module may be reproduced in any form without written permission from the copyright owners. Development Team of the Module Author: JOVY B. LICOS, MT-II Editor: SDO La Union, Learning Resource Quality Assurance Team Illustrator: Ernesto F. Ramos Jr., P II Management Team: Atty. Donato D. Balderas, Jr. Schools Division Superintendent Vivian Luz S. Pagatpatan, PhD Assistant Schools Division Superintendent German E. Flora, PhD, CID Chief Virgilio C. Boado, PhD, EPS in Charge of LRMS Rominel S. Sobremonte, Ed.D., EPS in Charge of Science Michael Jason D. Morales, PDO II Claire P. Toluyen, Librarian II General Chemistry 1 Quarter 2 – Week 1 Module 1- Quantum Numbers Target An electron in an atom is described in terms of four different quantum numbers. Three of the quantum numbers: n, ℓ, and m describes the atomic orbital. An atomic orbital may be thought of as the region of space around the nucleus where the probability of finding the electron with a given energy is greatest. It is commonly illustrated as an indistinct, cloud-like region, “thick” where the electron is most likely to be found “thin” where it will less likely be. In your previous lessons, you have learned about the atomic constitution of matter, that atoms are made up of elementary particles and these are: protons, neutrons and electrons. The arrangement of electrons in an atom is called its electronic structure. The electronic structure of an atom refers not only to the number of electrons that an atom possesses but also to their distribution around the nucleus and to their energies. Our knowledge of electronic structure is the result of one of the major development of the quantum theory. This learning material will provide you with information and activities that will help you understand the four quantum numbers that describe electrons and determine the magnetic property of the atom based on its electron configuration. After reading this learning material, you are expected to: Use quantum numbers to describe an electron in an atom (STEM_GC11ESIIa-b-54) Determine the magnetic property of the atom based on its electronic configuration (STEM_GC11ESIIa-b-57) Before going on, check how much you know about this topic. Answer the pretest on the next page and write your answer in ¼ sheet of paper. Pre- Test Direction: Select the letter of the correct answer and write in ¼ sheet of paper. 1. How many orientation does the S orbital can have? A. 1 B. 3 C. 5 D. 7 2. What quantum number describes the energy y of an orbital? A. angular (ℓ) C. principal B. magnetic (mℓ) D. spin (ms) A. Flexibility D. reactivity with water 3. Which of the following describes a diamagnetic atom? A. An atom where all the electrons are paired. B. An atom where some of the electrons are paired. C. An atom where none of the electrons are paired. D. An atom attracted to a magnetic field. 4. Which of the following describes a paramagnetic atom? A. An atom where all the electrons are paired. B. An atom where some of the electrons are unpaired. C. An atom are not attracted to a magnetic field. D. None of the above 5. Which of the following is the correct distribution of electrons of Sodium ( 11Na) electronic configuration? A. 1s2 2s2 2p6 3s1 C. 1s2 2s2 2p2 3s2 3d1 B. 1s2 2s2 2p5 3s1 D. 1s2 2s2 3s2 2p5 Jumpstart The question on how many quantum numbers are needed t describe any given system has no universal answer: for each system, one must find the answer by performing a full analysis of the system. Discover The Four Quantum Numbers That Describes Electrons Atomic orbitals are associated with characteristic energies, size, shapes, and orientation in space. These values assigned as the quantum numbers. 1. The principal quantum number (n), describes the energy of the electron. Designates the main energy level (floor) or shell. The energy of the electron is determined by its average distance from the nucleus or the principal energy level where it is. It can have integral values 1, 2, 3 and so forth. It also describes the CLOUD SIZE. The larger the value of n, the larger the cloud size. Energy levels closer to the nucleus have lower energy. As n increases, the orbital becomes larger and the electron spends more time farther from the nucleus. An increase in n also means that the electron has a higher energy and is therefore less tightly bound to the nucleus. The larger n is, the greater the average distance of an electron in the orbital from the nucleus and therefore the larger (and less stable) the orbital. The maximum number of electrons possible in a given shell is 2n2 . LEVEL MAXIMUM NO. OF ELECTRONS 2n2 n=1 n=2 n=3 n=4 n=5 n=6 n =7 2 8 18 32 50 72 98 In reality there are only 32 electrons in levels 5, 6 and 7 because of number of different elements discovered or man-made. 2. The azimuthal or angular momentum quantum number (ℓ), tells us the “shape” of the orbital. It designates the sublevel which the electron is said to occupy. It is also an integer, but its values are limited to a range of 0 to n-1. These values are: ℓ=0, for the electron in an orbital in the s sublevel ; ℓ=1 for the electron in an orbital in the p sublevel; ℓ=2 for the electron in an orbital in the d sublevel; and ℓ=3 when the electron occupies an orbital in f sublevel. ℓ Name of orbital 0 1 2 3 4 5______ s p d f g h The unusual sequence of letters (s,p,d) has a historical origin. Physicist who studied atomic emission spectra tried to correlate the observed spectral lines with the particular energy states involved in the transitions. They noted that some of the lines were sharp; some were diffuse or spread out; and some were very strong and hence referred to as principal lines. Subsequently, the initial letters of each adjective assigned to those energy states. However, after the letter d and starting with the letter f (for fundamental), the orbital designations follow alphabetical order. 3. The magnetic quantum number (mℓ) describes the orientation of the orbital in space. The number is also an integer, and its values are restricted to a range of +1 down through 0 to -1. When ℓ= 0, mℓ can have only one value: 0. This corresponds to a single s orbital which has a spherical shaped centered around the nucleus, as shown below. The spherical shape of the s orbital means that the electron is moving, with 90% probability in a region of space within the sphere, not around on the surface of the sphere. Like the other orbitals, the region of highest density in the s orbital roughly corresponds to its shape. When ℓ= 1, mℓ, has three values: +1, 0,-1 which corresponds to three p orbitals. Each orbital has “two lobes” in “dumbbell” shape that lie along three axes (x,y,z) as shown below: To distinguish these orbitals, they are named P x, Py, Pz.. When ℓ= 2, mℓ, five values: +2,+ 1,0,-1,-2. Four of the five kinds of d orbitals have clover-leaf shape but have different orientations. When ℓ= 3, mℓ, has seven values: +3,+2,+1,0,-1,-2,-3. These seven f orbitals have extremely complex shapes that are difficult to visualize. mℓ= 0 +1 s Px ℓ = 0 s orbital 0 -1 Py Pz 1 p orbital +2 +1 0 -1 -2 dyz dxy dxz dz2 dx2y2 2 d orbital +3 +2 +1 0 -1 -2 -3 3 f orbital 4. The spin quantum number (ms) refers to the “spin” of an electron in a given orbital. It can have only two values: arrow up ↑ is +1/2( referred to as “spin up”) and arrow down ↓ is -1/2 ( referred to as “spin down”). The spin of an electron can be one of two opposite directions, clockwise or counterclockwise. Since the spin quantum number has only possible values, it follows that an orbital can accommodate a maximum of two electrons only. The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states that no two electrons in the same atom can have identical values for all four of their quantum numbers. What this means is that no more than two electrons can occupy the same orbital, and that two electrons in the same orbital must have opposite spins. Because an electron spins, it creates a magnetic field, which can be oriented in one of two directions. For elements consisting of atoms without unpaired electrons, like Helium, 2He ( 1s2) and Argon, 18Ar ( 1s2 2s2 2p6 3s2 3p6) the spins are said to be paired. These elements are not attracted to magnets and are said to be diamagnetic. On the other hand, elements made up of atoms with unpaired electron like 8O (1s2 2s2 2p4) and Sodium, 11Na (1s2 2s2 2p6 3s1) are paramagnetic; that is, they are attracted to magnetic field. Writing Electron Configuration of Atoms Where are electrons supposed to be found in the atom? How is the arrangement of electrons in an atom described, known, and shown? The distribution of electrons among the orbitals of an atom is called the electron configuration. The electrons are filled in according to a scheme known as the Aufbau principle (“building-up”), which corresponds (for the most part) to increasing energy of the subshells: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f. It is not necessary to memorize this listing, because the order in which the electrons are filled in can be read from the periodic table in the following fashion: IA 1s 1 VIII A II A III A IV A V A VI A VII A 2 2s 2p 3 3s 3p 4 4s 3d 4p 5s 4d 5p 6s 5d 6p 7s 6d 5 6 1s 7 n (n)p (n)s (n-1)d (n-2)f In electron configurations, write in the orbitals that are occupied by electrons, followed by a superscript to indicate how many electrons are in the set of orbitals (e.g., H 1s1) Another way to indicate the placement of electrons is an orbital diagram, in which each orbital is represented by a square (or circle), for example 1H (1s1) , He 2 and the electrons as arrows pointing up or down (indicating the electron spin). When electrons are placed in a set of orbitals of equal energy, every orbital in a sublevel is singly occupied before any orbital is doubly occupied. (Hund’s rule). Example; Nitrogen atoms, 7N (1s2 2s2 2p3) All of the electrons in singly occupied orbitals have the same spin (to maximize total spin). In a ground state configuration, all of the electrons are in as low an energy level as it is possible for them to be. When an electron absorbs energy, it occupies a higher energy orbital, and is said to be in an excited state. Explore Here are some enrichment activities for you to strengthen the basic concepts you have learned from this lesson Activity 1. Complete Me! Electron configuration for atoms of the first 10 elements. Atom Electronic Configuration Atom 19K 15P 26Fe 25Mn 20Ca 14Si 17Cl Electronic Configuration 11Na 21Sc 10Ne Activity 2. Try Me! The arrangement of electrons in an atom can also be represented by orbital diagram. Show by orbital diagram the electron configuration of the following atoms. 1. 18Ar 2. 10Ne 3. 19k 4. 7N 5. 15P Activity 3. Let’s Do It! Write first the electron configuration of the following atoms, then identify the magnetic property based on the electron configuration. Atoms 1. 8O 2. 11Na 3. 29Cu 4. 25Mn 5. 2He Electron Configuration Magnetic Property (Paramagnetic/Diamagnetic) You are really doing great! That’s amazing! Deepen At this point, continue assessing your learning by answering the activity below. Activity 1. “The Reason is Configuration”. A. When the iron (Fe) is used as a component of objects like hair pins, needles, and paper clips, the objects are attracted by magnets. 1. Prove that the presence of iron (Fe) in them is the cause of the magnetic property by writing the electron configuration of 26Fe. ________________________________________________________________ _________________________________________________________________ 2. How many unpaired electrons does Fe have? ____________________ 3. What type of magnetic property is described in the electron configuration of iron? Gauge Direction: Read carefully each question. Use a separate sheet of paper for your answer. Write the letter of the best answer.(15 pts) 1. Which of the following defines the spin quantum number? A. The orientation or shape of the orbital the electron is in. B. The orientation of orbitals around the nucleus. C. The energy level the electron is in? D. The direction of electron spin. 2. The magnetic quantum number (mℓ) refers to? A. the orientation or shape of the orbital the electron is in. B. the orientation of orbitals around the nucleus. C. the energy level the electron is in? D. the direction of electron spin. 3. The angular quantum number (ℓ)refers to? A. the orientation or shape of the orbital the electron is in. B. the orientation of orbitals around the nucleus. C. the energy level the electron is in? D. the direction of electron spin. 4. Which of the following defines the principal quantum number (n)? A. the orientation or shape of the orbital the electron is in. B. the orientation of orbitals around the nucleus. C. the energy level the electron is in? D. the direction of electron spin. 5. An orbital n=2 can hold up to how many electron? A. 2 electrons C. 11 electrons B. 8 electrons D. 18 electron. 6. An electron in an f sublevel can have the principal quantum number of _______.? A. 1 B. 2 C. 3 D. 4 7. How many orientation does the S orbital can have? A. 1 B. 3 C. 5 D. 7 8. What quantum number describes the shape of an orbital? A. angular (ℓ) C. principal B. magnetic (mℓ) D. spin (ms) 9. Which of the following is the correct electron configuration of oxygen atom? A. 1s2 2s1 2p3 C. 1s2 2s2 2p6 B. 1s2 2s2 2p4 D. 1s2 2s2 3p4 10. Which of the following explains Pauli Exclusion Principle? A. An atomic orbital can only hold a maximum of two electrons each with opposite direction. B. An atomic orbital can hold a maximum of 6 electrons each with the same spin. C. An atomic orbital can only hold a minimum of two electrons each with opposite direction. D. An atomic orbital can hold a maximum of 6 electrons each with opposite spin. 11. Which of the following describes a diamagnetic atom? A. An atom where all the electrons are paired. B. An atom where some of the electrons are paired. C. An atom where none of the electrons are paired. D. An atom attracted to a magnetic field. 12. Which of the following electron configuration is an example of diamagnetic atom? A. 1s2 2s2 2p4 C. 1s2 2s2 2p6 3s2 3p6 4s2 3d9 B. 1s2 2s2 2p6 3s1 D. 1s2 2s2 2p6 3s2 13. Which of the following electron configuration is an example of paramagnetic atom? A. 1s2 C. 1s2 2s2 2p6 3s2 3p6 4s2 3d9 B. 1s2 2s2 2p6 3s2 D. 1s2 2s2 2p6 3s2 14. The word Aufbau means building up in German. Which of the following describes Aufbau Principle? A. electrons with the same spin cannot occupy the same orbital. B. Since electrons repel each other, electrons will occupy single orbitals within and energy level before doubling up. C. electrons fill lower energy levels first before occupying higher energy levels. D. None of the above. 15. Which of the following shows the correct distribution of electrons of Oxygen in atomic orbitals? A. B. C. D. Gauge 1. 2. 3. 4. 5. D A B C B 6. D 7. A 8. A 9. B 10. A 11. 12. 13. 14. 15. A D C C A Pre-test 1. 2. 3. 4. 5. A C A B A EXPLORE: Activity 1.Complete Me! 1.19K - 1s2 2s2 2p6 3s2 3p6 4s1 2.25Mn - 1s2 2s2 2p6 3s2 3p6 4s2 3d5 3.26Fe – 1s2 2s2 2p6 3s2 3p6 4s2 3d6 4.20Ca – 1s2 2s2 2p6 3s2 3p6 4s2 5.14Si – 1s2 2s2 2p6 3s2 3p2 Activity 2: Try 15P 5. 7N 1. 2. 3. 4. 6.15P - 1s2 2s2 2p6 3s2 3p3 7.17Cl - 1s2 2s2 2p6 3s2 3p5 8.11Na - 1s2 2s2 2p6 3s1 9. 21Sc -1s2 2s2 2p6 3s2 3p6 4s2 3d1 10.10Ne - 1s2 2s2 2p6 Me! 18Ar 10Ne C 19K Activity 3: Let’s Do It! 1. 8O 2. 11Na3. 29Cu – 4. 25Mn 5. 2He - 1s2 1s2 1s2 1s2 1s2 2s2 2s2 2s2 2s2 2p4 2p6 3s1 2p6 3s2 3p6 4s2 3p6 3d3 2p6 3s2 3p6 4s2 3p5 paramagnetic paramagnetic paramagnetic paramagnetic diamagnetic ANSWER KEY References A. Books Cervantes, Charry Vida R. and Dizon, Reynald D. General Chemistry 1 for Senior Highj School (Specialized Subjects for STEM Strand). Manila, Philippines: LORIMAR Publishing Inc.2008. Ilao, Lacsamana V., Betty M.Lontoc, and Edwehna Elinore S. Paderna-Gayon. General Chemistry 1. Sampaloc, Manila: Rex Bookstore, Inc. 2016. Reyes, Armida Bernaldo et. al. General Inorganic and Organic Chemistry for Health-Related Programs. Dagupan City, Philippines: SLA Publishing House, 2008. B. Website https://www.angelo.edu/faculty/kboudrea/general/quantum_numbe rs/Quantum_Numbers.pdf https://www.riverdell.org/cms/lib05/NJ01001380/Centricity/Domai n/98/quantum%20numbers%20tutorial%20and%20practice.pdf https://reviewgamezone.com/mc/candidate/test/?test_id=34542&titl e=Quantum%20Numbers https://www.chemicool.com/definition/pauli-exclusionprinciple.html https://socratic.org/questions/55d55b3b581e2a3e8fbccd81 https://study.com/academy/practice/quiz-worksheet-diamagnetismparamagnetism.html https://www.britannica.com/science/orbital https://www.liverpool.ac.uk/~ngreeves/ltfdemo/jmol/p-orbitals.htm https://www.qsstudy.com/chemistry/explain-shape-d-orbitals