Specific Heat Calculations: Student Activity Sheet
advertisement

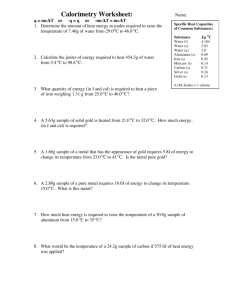
Specific Heat Q=mcΔT Student Activity Sheet HS PS 3-1 ____________________________________________________________________________________________________________________________________________________________________________________ Essential Question: How can we predict temperature changes of different substances? Use the following data tables to answer the questions below. Substance Specific Heat Capacity (J/g℃) Useful Equations: q = mcΔT (q = heat, m = mass, c = specific heat, ΔT = change in temp) water 4.184 J/g℃ silicon 0.703 J/g℃ ΔT = Tfinal - Tinitial copper 0.387 J/g℃ TC = 5/9 * (Tf - 32) gold 0.129 J/g℃ TK = TC + 273 aluminum 0.89 J/g℃ 1 kg = 1000 g iron 0.45 J/g℃ silver 0.24 J/g℃ lead 0.128 J/g℃ 1 cal = 4.184 J 1 kcal = 1000 cal For each question, underline what is given and circle what you are trying to solve for. Example: How much heat would 200 grams of water have to absorb to raise the temperature by 10℃? -write what is given: m = 200 g ΔT = 10℃ c = 4.184 J/g℃ (find in the data table) -write the equation: q = mcΔT -input given units: q = (200 g)*(4.184 J/g℃)*(10℃) -solve for the unknown: q = 8368 J ***remember to always include correct units! q = joules, m = grams, ΔT = ℃, c = J/g℃) 1. A 150 g sample of iron is heated from 15℃ to 100℃. How much heat did the sample gain? Answer: 2. How much will the temperature change if a 126.3 gram sample of silver loses 1000 J of heat? Answer: Specific Heat Q=mcΔT Student Activity Sheet HS PS 3-1 ____________________________________________________________________________________________________________________________________________________________________________________ 3. Calculate how much energy would be needed to heat a beaker of water at 18℃ to boiling. The mass of the water is 15 grams. Answer: 4. An unknown amount of gold was heated with 40 J and the temperature increased from 25.8℃ to 42.3℃. What is the mass of the gold? Answer: 5. How much energy is lost when a 524 gram copper statue is cooled from 40℃ to 16.7℃? Answer: 6. An 18.2 gram sample of silicone is exposed to 100 joules of energy. What is the final temperature if the sample was initially at 25℃? Answer: 7. A sample of iron was heated from 37.2℃ to 91.2℃, which required 40 calories. What was the mass of the sample? Answer: 8. A 39 gram unknown sample was exposed to 100 J of heat, causing it to raise in temperature from 23℃ to 43℃. What is the specific heat of the metal and determine the identity. Answer: Specific Heat Q=mcΔT Student Activity Sheet HS PS 3-1 ____________________________________________________________________________________________________________________________________________________________________________________ Answer Key: 1. 5,737.5 J 2. 33.0℃ 3. 5,146.3 J 4. 18.8 g 5. 4725 J 6. 32.8℃ 7. 1.64 g 8. 0.128 J/g℃, lead