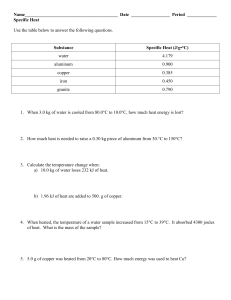
1°Celsius
advertisement

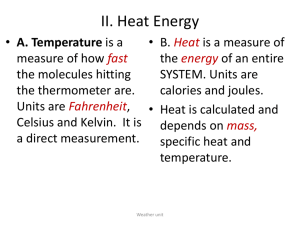
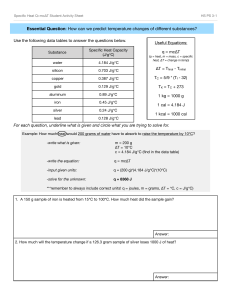
Specific Heat Capacity What’s a property? • It’s a characteristic of a given substance • All substances of the same type will share these characteristics. • They are often very useful if you need to identify an unknown substance • Examples? One essential property of all substances is called What is it? • It’s the amount of energy that’s required to raise the temperature of one gram of a substance by 1°Celsius • Every substance has it’s own specific heat capacity. For example... Substance Air Aluminium Copper Gold Iron Mercury NaCl Ice Water J/g °C 1.01 0.9 0.38 0.13 0.45 0.14 0.86 2.03 4.18 What does this mean? • You need 4 joules of energy to raise the temperature of a gram of water (about a mL) by one degree. • BUT you only need 1 joule to raise the temperature of a gram of air by the same amount. • This explains why air heats up and cools down so much faster than water. Calculating specific heat capacity Q = Energy in joules c = specific heat capacity m=Mass ∆t = the temperature change OR Tfinal - Tinitial Further reading: p. 427-431 Assignment: Using the information in your texts, invent 3 specific heat problems for each of types 1, 2 and 3 1. Don’t know Q • You leave a glass containing 500g of water outside on a sunny day. When the water came out of the fridge it had a temperature of 5°C. After an hour, it’s temperature has risen to 20°C. You know from science class that the specific heat of water is 4.18J/g°C. How much energy was added to the water? 2. Don’t know m • You are working with a piece of iron in the lab, but the scale is broken so you can’t find the mass. You have been heating the metal. You know that you have added 2000J of energy and the temperature of the iron has increased by 100°C. If the specific heat is 0.45J/g°C, find the mass 3- Don’t know c • You have 100g of an unknown substance. You have added 2030J of energy to the sample and the temperature has risen 10°C. What is the specific heat of the sample? 4- Don’t know ∆t • You apply 3000J of energy to a sample of aluminum which has a mass of 50g. How much will the temperature increase if the specific heat capacity of aluminum is 0.9J/g °C