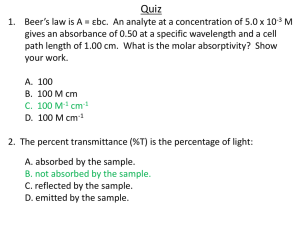
Spectrophotometry MBLG1001 second session Abs = 0.51 Introduction Many compounds absorb ultraviolet (UV) or visible (Vis.) light. The diagram below shows a beam of monochromatic radiation of radiant power P0, directed at a sample solution. Absorption takes place and the beam of radiation leaving the sample has radiant power P. The amount of radiation absorbed may be measured in a number of ways: Transmittance, T = P / P 0 % Transmittance, %T = 100 T Absorbance, A = log10 P0 / P A = log10 1 / T A = log10 100 / %T A = 2 - log10 %T Abs = log(I0/IT) 10Abs = (I0/IT), inverting 10-Abs = (IT/I0), 100* 10-Abs = 100*(IT/I0), 102-Abs = %T ln Io/I = ax or log Io/I = a/2.303 (x)…… 4 Log Io/I = a’ (x) is called extinction coefficient Look at slide 2 log Io/I is the absorbance. This is the Lambert law The law is extended by Beer, dI/dx is proportional to concentration c and dI/dx is proportional to I and c, dI/dx = bcx b is the molar absorption coefficient, b is a constan. If c is in mol/L b will be in molar absorption coefficient . log Io/I = b/2/303 cx log Io/I =bcx and bcx is the absorbance (A) log Io/I = A Transmittance to Absorbance The amount of radiation absorbed can be measured by the transmittance. The relation between transmittance (T) and absorbance (A) is given by the Beer-Lambert law (Beer’s law). According to Beer’s Law, the absorbance is formulated as: • The last equation, A = 2 - log10 %T , is worth remembering because it allows you to easily calculate absorbance from percentage transmittance data. • The relationship between absorbance and transmittance is illustrated in the following diagram: • • So, if all the light passes through a solution without any absorption, then absorbance is zero, and percent transmittance is 100%. If all the light is absorbed, then percent transmittance is zero, and absorption is infinite. Limitations of the Beer-Lambert law The linearity of the Beer-Lambert law is limited by chemical and instrumental factors. Causes of nonlinearity include: •deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity •scattering of light due to particulates in the sample •fluoresecence or phosphorescence of the sample •changes in refractive index at high analyte concentration •shifts in chemical equilibria as a function of concentration •non-monochromatic radiation, deviations can be minimized by using a relatively flat part of the absorption spectrum such as the maximum of an absorption band •stray light Construction of Spectrophotometry The Spectrophotometer Photomultiplier Tube (PMT) Introduction Photomultiplier Tubes (PMTS) are light detectors that are useful in low intensity applications such as fluorescence spectroscopy. Due to high internal gain, PMTs are very sensitive detectors. Design PMTs are similar to phototubes. They consist of a photocathode and a series of dynodes in an evacuated glass enclosure. Photons that strikes the photoemissive cathode emits electrons due to the photoelectric effect. Instead of collecting these few electrons (there should not be a lot, since the primarily use for PMT is for verly low signal) at an anode like in the phototubes, the electrons are accelerated towards a series of additional electrodes called dynodes. These electrodes are each maintained at a more positive potential. Additional electrons are generated at each dynode. This cascading effect creates 105 to 107 electrons for each photon hitting the first cathode depending on the number of dynodes and the accelerating voltage. This amplified signal is finally collected at the anode where it can be measured. Schematic of a PMT • Cross section of a photomultiplier tube Schematic of a wavelength-selectable, single-beam UV-Vis spectrophotometer Before the prac class begins experimental work…. • The spectrophotometer actually measures transmittance: %T = (IT/I0)*100. • This is the intensity of light transmitted through the solution (IT) divided by the intensity of light entering the solution, the incident light (I0). The problem with this measurement is that as the solution becomes more concentrated the %T decreases. The relationship is also not linear. • Absorbance is actually log10I0/IT Now, suppose we have a solution of copper sulphate (which appears blue because it has an absorption maximum at 600 nm). We look at the way in which the intensity of the light (radiant power) changes as it passes through the solution in a 1 cm cuvette. We will look at the reduction every 0.2 cm as shown in the diagram below. The Law says that the fraction of the light absorbed by each layer of solution is the same. For our illustration, we will suppose that this fraction is 0.5 for each 0.2 cm "layer" and calculate the following data: Question : What is the significance of the molar absorbtivity, e ? Answer : To begin we will rearrange the equation A = εbc : ε = A / bc In words, this relationship can be stated as "ε is a measure of the amount of light absorbed per unit concentration". Molar absorbtivity is a constant for a particular substance, so if the concentration of the solution is halved so is the absorbance, which is exactly what you would expect. Let us take a compound with a very high value of molar absorbtivity, say 100,000 L mol-1 cm-1, which is in a solution in a 1 cm pathlength cuvette and gives an absorbance of 1. Therefore, c = 1 / 100,000 = 1 ´ 10-5 mol L-1 Now let us take a compound with a very low value of e, say 20 L mol-1 cm-1 which is in solution in a 1 cm pathlength cuvette and gives an absorbance of 1. e = 1 / 1 ´ c Therefore, c = 1 / 20 = 0.05 mol L-1 Question : What is the molar absorbtivity of Cu2+ ions in an aqueous solution of CuSO4 ? It is either 20 or 100,000 L mol-1 cm-1 Answer : I am guessing that you think the higher value is correct, because copper sulphate solutions you have seen are usually a beautiful bright blue colour. However, the actual molar absorbtivity value is 20 L mol-1 cm-1 ! The bright blue colour is seen because the concentration of the solution is very high. b-carotene is an organic compound found in vegatables and is responsible for the colour of carrots. It is found at exceedingly low concentrations. You may not be surprised to learn that the molar absorbtivity of b-carotene is 100,000 L mol-1 cm-1 ! The principle of the UV absorbance method is that nucleic acids (DNA or RNA) contain conjugated double bonds in their purine and pyrimidine rings that have a specific absorption peak at 260 nm. The maximum absorbance of nucleic acids occurs at a wavelength of 260 nm Proteins in solution absorb ultraviolet light with absorbance maxima at 280 and 200 nm. Amino acids with aromatic rings are the primary reason for the absorbance peak at 280 nm. Due to the presence of tyrosine and tryptophan, proteins and peptides containing these aromatic amino acids absorb UV light at a wavelength of 280 nm. Each of these residues has distinct absorption and emission wavelengths and varies in quantum yields. Phenylalanine and disulfide bonds also contribute in the absorption at this wavelength but since it is relatively insignificant, it can only be observed in the absence of both tryptophan and tyrosine. At a wavelength of 260 nm, the average extinction coefficient for doublestranded DNA is 0.020 (μg/ml)−1 cm−1, for single-stranded DNA it is 0.027 (μg/ml)−1 cm−1, for single-stranded RNA it is 0.025 (μg/ml)−1 cm−1 and for short single-stranded oligonucleotides it is dependent on the length and base composition. A=εbc 1=0.02x1xc C=1/0.02x1 = 50 µg/ml A 260/280 ratio of ~1.8 is generally accepted as “pure” for DNA; a ratio of ~2.0 is generally accepted as “pure” for RNA. Abnormal 260/280 ratios usually indicate that a sample is contaminated by residual phenol, guanidine, or other reagent used in the extraction protocol, in which case the ratio is normally low. Relationship between %Transmittance and light path length and concentration %T 100 100 80 80 60 %T 60 40 40 20 20 0 length 0 concentration Transmittance = IT/I0 = e-acl where a is an extinction constant, c is concentration and l is light path length Absorbance increases linearly with concentration From IT/I0 = e-acl I have used a here to describe a constant that is proportional to the extinction coefficient. As you can see from the maths it is not the mMolar extinction coefficient taking logs of both sides and inverting Ln (I0/IT) = acl Converting to Log10 Log10(I0/IT) = ecl Hence the Beer-Lambert Law A = ecl Before the prac class begins experimental work…. • Absorbance increases linearly with concentration as predicted by the BeerLambert Law A = ecl • Explain why the working range of a spectrophotometer is 0.1 – 1.0. Remember Abs is a log scale. An absorbance of 1.6 is 2% light transmitted while an absorbance of 2 is 1% light transmitted. The class specs can not accurately distinguish 1% from 2%. An absorbance of 1.0 is 10% transmitted light. Experiment 1: Identifying a compound by spectrophotometry • If a compound absorbs light its absorption spectrum is a unique property of that compound. • The molecular structure is responsible for the absorption properties • The most common feature of absorbing compounds are conjugated double bonds, often as an aromatic ring Experiment 1: Identifying a compound by spectrophotometry • This amount of absorbed energy (DE) will determine the l of light absorbed. • The DE is inversely proportional to the wavelength of light absorbed ie. DE = hc/l, where h is Planck’s constant and c is the velocity of light. (Remember this from physics!??) Common Absorbing Biochemicals O • The bases of nucleic acids N NH2 O N H3C N H N H Thymine O N H N NH2 Guanine N NH NH NH2 N N Adenine N H Cytosine O Nucleic Acid Absorption Properties e (mM-1cm-1) Guanine lmax (nm) 275 Adenine 260 12.9 Cytosine 265 5.8 Thymine 258 8.0 Base 8.0 Absorption Spectrum: Guanine Absorption Spectrum: Adenine 0.5 1.0 0.4 0.8 Absorbance Absorbance Nucleic Acid Absorption Properties 0.3 0.2 0.1 0 220 240 260 280 300 0.6 0.4 0.2 0.0 220 320 Wavelength (nm ) 0.4 0.5 Absorbance Absorbance 0.6 0.3 0.2 0.1 260 280 Wavelength (nm ) 280 300 320 Absorption Spectrum: Thymine 0.5 240 260 Wavelength (nm ) Absorption Spectrum: Cytosine 0.0 220 240 300 320 0.4 0.3 0.2 0.1 0.0 220 240 260 280 300 Wavelength (nm ) 320 Amino Acid absorption Properties H2N CH C O O O H2N OH CH C H2N OH C CH2 CH2 CH2 CH N NH HN Histidine OH Tryptophan Tyrosine O H2N CH C CH2 OH O H2N CH C CH2 SH Cysteine Phenylanine OH OH The Dyes • The dyes chosen for this experiment are different colours (A – F) • Each pair of students will be assigned a dye by the demonstrator. They use this dye for the whole practical. • Students should take note of the colour of their dye and record the concentration (mM) on the bottle Obtaining a Spectrum for the dye. • Using the Shimadzu spectrophotometers in spectrum mode (mode 2 on main menu) place a 1 mL plastic cuvette full of H2O in the holder and obtain a baseline correction (F1). This will take some time. • Then, using the same cuvette, fill it with the dye solution and obtain a spectrum. Find the peaks. If you are unclear how to do this practise beforehand. Obtaining a Spectrum for the dye. • The reason for doing this is to find the absorbing region of the dye. It takes a long time to obtain a spectrum from 600 nm to 350 nm. A quick narrowing of the range is to be encouraged. • Get students to consider the colour of the solution and how this might give clues to the absorption minima and maxima The relationship between colour and absorption • A compound will be yellow if it reflects light in the yellow wavelengths and absorbs light of other wavelengths. • Yellow compounds (often red crystals) usually absorb in the blue range ~450 – 350 nm and have an absorption minimum >550 nm Dye C: Riboflavin Absorption Spectrum: Dye C 1.2 Dye C is yellow red and has an absorption minimum in the yellow/red region Absorbance 1.0 0.8 0.6 0.4 0.2 0.0 350 400 450 500 w avelength (nm ) 550 600 Obtaining the spectrum • Once the baseline is corrected and the absorbing range determined, find the absorbance of the dye every 10 nm within the absorbing range. Every 50 nm will do outside the absorbing range. • If the baseline correction is done you don’t have to zero every time you change wavelengths. This saves heaps of time. Obtaining the spectrum • Students must, by the next lab, plot the spectrum. It would be a good idea to get them to this now if there are free computers. Otherwise get them to identify the dye by the peaks, comparing to the sample spectra at the back of this section of the lab manual. • From the concentration on the bottle estimate the extinction coefficient. Obtaining the spectrum • Make sure you are very familiar with the Excel chart drawing process as you will need to help the students here. Practise with the spectro.xls spreadsheet provided. It has the raw data obtained for riboflavin and the worked solution. • Check out what is expected graph-wise in the worked solution. Students should have practised much of this with the Excel task in the last practical. Calculating the Extinction Coefficient • This comes directly from the relationship A = ecl, Where e is the extinction coefficient expressed in the units of c, the concentration. In this experiment the conc units will be mM so e will have the units mM-1cm-1. Round the value off to 1 dec pl. Discussion • Predict which of the following parameters would change with dilution? How would they change? • The number of peaks • The l max • Al1/Al2 • Absorbance at l max • Extinction Coefficient • Transmittance at lmax • Can you predict what would happen to the absorption spectrum if you diluted your dye with another dye? Experiment 2: The Standard Curve • Although identifying a compound by spectro is a useful property, spectrophometry is used more often to measure the concentration of a compound. • Sometimes the extinction coefficient can be used directly. This occurs when the compound of interest has an intrinsic absorbance. Experiment 2: The Standard Curve • If the compound of interest does not have its own intrinsic absorbance then a coloured derivative must be made by reacting it with reagents. Then a standard curve must be produced. • In today’s practical students will gain experience at producing and using a standard curve, even though in this situation you would normally use the extinction coefficient. Experiment 2: The Standard Curve • Using the same solution as the one used to obtain the spectrum, get the students to dilute it so that there are at least 5 points for the line. My suggestion is 200, 400, 600, 800, 1000 uL of dye, then make each up to 1 mL with water. • Mix well and obtain the absorbances at the lmax. The standard curve Absorbance @ 445 nm Standard Curve: Riboflavin 1.2 y = 0.0125x 1.0 R = 0.9999 2 0.8 0.6 0.4 0.2 0.0 0 20 40 60 [Riboflavin] (nmol/mL) 80 100 The standard curve Absorbance @ 445 nm Standard Curve: Riboflavin 1.2 y = 0.0125x 1.0 R = 0.9999 2 0.8 Plot the concentration in nmol/tube or nmol/mL 0.6 0.4 0.2 0.0 0 20 40 60 [Riboflavin] (nmol/mL) 80 100 The standard curve • The main point of confusion in this task is how to plot the concentration. The purists would plot it in mM or uM and this would be correct BUT confusing for the students when they come to back calculate with it. • Instead plot it in nmoles per tube which in this case is nmol/mL. Note that the riboflavin concentration range is ~0 – 80 nmol/mL. It is not “neat” due to the concentration of the starting dye solution 0f 0.0836 mM. How to use the standard curve • The standard curve is used to find the concentration of an unknown solution of riboflavin. • This practical session has 2 different unknowns the students must determine the concentration of; one which is in the working range of the spectrophotometer or standard curve i.e. 0.1 – 1.0, the other is outside the range. Unknown 3a • This unknown can be directly determined by measuring its absorbance without dilution. • However it is always good practise to do at least one dilution when estimating a concentration. • The obvious dilution is a 1 in 2 dilution. This is your chance to introduce dilutions to the students. Dilutions • A 1 in 2 dilution is 1 part riboflavin unknown C1 and 1 part H2O. • If you wanted to make up a 1 in 2 dilution of unknown C1 which could be easily read off the standard curve you would make it up to 1 mL. • This would mean 500 uL of unknown C1 and 500 uL H2O. Experiment 3a:Unknown C1 Slope 0.01245 Intercept 0.000571 Unknown C1 Dilution factor 1 2 A445 0.829 0.417 [Riboflavin] (nmol/mL) 66.5 33.4 Original Conc. (nmol/mL) 66.5 66.9 Average (nmol/mL) 66.7 From standard curve or using INTERCEPT function in Excel From standard curve or using SLOPE function in Excel Slope 0.01245 Intercept 0.000571 Unknown C1 Dilution factor 1 2 A445 0.829 0.417 [Riboflavin] (nmol/tube=mL) 66.5 33.4 Original Conc. (nmol/mL) 66.5 66.9 Average (nmol/mL) 66.7 Average of 2 values [Riboflavin] (nmol/mL) = (A445intercept)/slope [original] = [riboflavin]* dilution factor Quick tips • You can use the extinction coefficient obtained in the first experiment or the standard curve. Get the students to try both methods. • To get from the Absorbance to the concentration you solve the equation of the standard curve for x; you know the y value (Abs) and you want to find out the x value (conc.) Why we express the concentration in nmol/tube • Provided you make the unknown dilutions to the same volume as the standards you can directly work out how much there is in the tube straight from the graph. • In the next exercise, unknown C2 or C3, it will be a real advantage Discussion Q from this section • Where does the extinction coefficient fit in to the std curve? – It is the gradient, but the units of the ext. coefficient are in the conc. Units on the x-axis • What would happen to the absorbance response and the equation of the line if: – you measured the absorbance at a wavelength other than the lmax? – It would be linear but the gradient i.e. the ext. coefficient would be lower See the varyQ worksheet in the spectro.xls What would happen to the absorbance response and the equation of the line if: • you expressed the concentration in different units (try M and % (w/v), obtaining the molecular weight of the dye from your demonstrator)? – The equation changes, in particular the gradient. The easiest way is to try this. Use the data given in the spectro spreadsheet. – you made the dye solutions up in 3 mL instead of 1 mL? – No difference, the absorbance is dependent on the concentration, which is volume independent. The only problem is if the cuvette is not full enough to cover light source – you used cuvettes with a 2 cm light path instead of 1 cm? The slope would be double as the absorbance would double for each tube Experiment 3b: Unknown outside the working range of the spectrophotometer • What concentration of riboflavin gives an absorbance of 0.5? (always aim for the middle of the range) Using A = ecl……0.5 = 12.5*c*1 Conc = 0.04 mM à 40 uM à 40 nmol/mL • So we need 40 nmoles of riboflavin in 1 mL to get an absorbance of 0.5 Experiment 3b: • Now how much of our unknown do we need to add to give an absorbance of 0.5? • Our unknown, C2, lies between 0.5 and 1 mM. Undiluted this unknown would give an absorbance between 6 and 12…way too high! • So we need to dilute our unknown…but by how much Experiment 3b: • Let’s consider the upper end of the range, 1 mM. • If our unknown is 1 mM, which is 1 umol/mL or 1 nmol/uL then….. • as we need 40 nmol/mL to give an absorbance of 0.5 so we would need to add 40 uL (40/1). Experiment 3b: • Then let’s consider the lower end of the range, 0.5 mM. • If our unknown is 0.5 mM, which is 0.5 umol/mL or 0.5 nmol/uL then….. • as we need 40 nmol/mL to give an absorbance of 0.5 so we would need to add 80 uL (40/0.5). Experiment 3b: • So we need to add between 40 and 80 uL of our unknown and make these up to 1 mL à mix well ……. • Then measure the absorbances of our samples. 0.396 0.490 0.594 0.681 0.774 Results Unknown C2 Range (0.5 - 1.0 mM) Unknown (uL) A445 [Riboflavin] (nmol/mL) [Riboflavin] (nmol/uL) [Riboflavin] (mM) 40 0.396 50 0.49 60 0.594 70 0.681 80 0.774 31.76 39.31 47.66 54.65 62.12 0.79 0.79 0.79 0.78 0.78 0.79 The back calculations • The volume of the unknown (uL) and the A445 are entered in directly as data. • To calculate the [Riboflavin] (nmol/mL) you solve the standard curve equation for x. This gives the #nmoles of riboflavin in each cuvette. 31.76 39.31 47.66 54.65 62.12 The back calculations From the [Riboflavin] (nmol/mL) we need to know the concentration in the original unknown C2 31.76 39.31 47.66 54.65 ? 62.12 The back calculations Each cuvette has a known volume of the original unknown added and we know #nmoles of riboflavin in each cuvette 40 uL 50 uL 60 uL 70 uL 80 uL 31.76 nmoles 39.31 nmoles 47.66 nmoles 54.65 nmoles 62.12 nmoles If we simply divide the #nmoles by the volume added in uL we have the original concentration of the unknown riboflavin in nmol/uL which is mM. After all we only added two solutions to each cuvette; water and riboflavin. We hope the nmoles came from the dye not the water. 40 uL 0.79 mM 50 uL 60 uL 70 uL 80 uL 0.79 mM 0.79 mM 0.78 mM 0.78 mM Average = 0.79 mM For unknown C3: range 1 – 2 mM • The upper range: 2 mM à 2 umol/mL à 2 nmol/uL • So we need to add 40/2 = 20 uL • The lower range: • 1 mM à 1 nmol/uL • So we need 40/1 = 40 uL Results Unknown C3 (1 - 2 mM) Unknown (uL) A445 [Riboflavin] (nmol/mL) [Riboflavin] (nmol/uL) [Riboflavin] (mM) 20 25 30 35 40 0.443 0.539 0.657 0.755 0.869 35.54 43.25 52.72 60.59 69.75 1.78 1.73 1.76 1.73 1.74 1.75 Integrating this equation from z = 0 to z = b gives: or Since N (molecules/cm3) * (1 mole / 6.023x1023 molecules) * 1000 cm3 / liter = c (moles/liter) and 2.303 * log(x) = ln(x), then or where = * (6.023x1020 / 2.303) = * 2.61x1020 Typical cross-sections and molar absorptivities are: (cm2 (M-1 cm- ) 1) absorption - atoms 10-12 3x108 absorption - molecules 10-16 3x104 absorption - infrared 10-19 3x10 Raman scattering 10-29 3x10-9 Double Beam UV.VIS Spectrophotometer diagram of a double-beam UV-Vis. spectrophotometer Question : What is the significance of the molar absorbtivity, e ? Answer : To begin we will rearrange the equation A = e bc : e = A / bc In words, this relationship can be stated as " e is a measure of the amount of light absorbed per unit concentration". Molar absorbtivity is a constant for a particular substance, so if the concentration of the solution is halved so is the absorbance, which is exactly what you would expect. Let us take a compound with a very high value of molar absorbtivity, say 100,000 L mol-1 cm-1, which is in a solution in a 1 cm pathlength cuvette and gives an absorbance of 1. e=1/1xC Therefore, c = 1 / 100,000 = 1 x 10-5 mol L-1 Now let us take a compound with a very low value of e, say 20 L mol-1 cm-1 which is in solution in a 1 cm pathlength cuvette and gives an absorbance of 1. e=1/1xc Therefore, c = 1 / 20 = 0.05 mol L-1 The answer is now obvious - a compound with a high molar absorbtivity is very effective at absorbing light (of the appropriate wavelength), and hence low concentrations of a compound with a high molar absorbtivity can be easily which means high Sensitivity Crystal Violet Absorption Spectrum 1 .4 Absorbance 1 .2 1 0 .8 0 .6 lmax 0 .4 0 .2 0 200 250 30 0 35 0 400 450 500 wavelength, nm 550 600 650 700 750