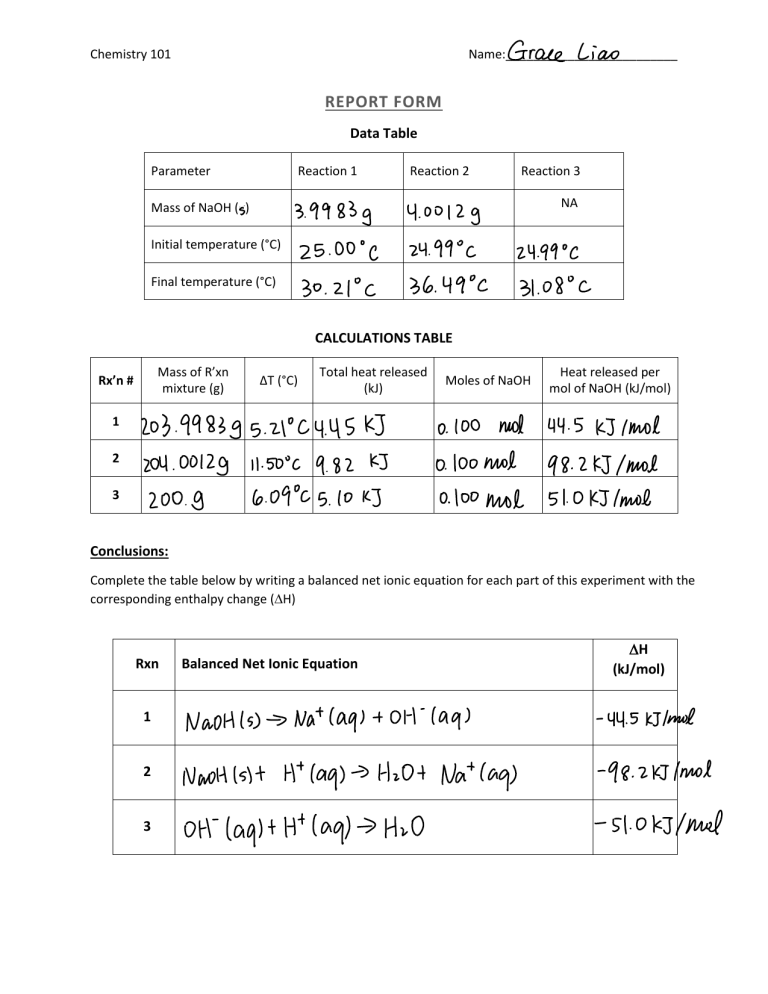
Chemistry 101 Name:_________________________ REPORT FORM Data Table Parameter Reaction 1 Reaction 2 Reaction 3 NA Mass of NaOH (g) Initial temperature (°C) Final temperature (°C) CALCULATIONS TABLE Mass of R’xn mixture (g) Rx’n # ΔT (°C) Total heat released (kJ) Moles of NaOH Heat released per mol of NaOH (kJ/mol) 1 2 3 Conclusions: Complete the table below by writing a balanced net ionic equation for each part of this experiment with the corresponding enthalpy change ( H) Rxn 1 2 3 Balanced Net Ionic Equation H (kJ/mol) Calculations: 1. Determine the change in temperature, ΔT, for each reaction. Record your results in the results table in Step 15. 2. Calculate the mass of the reaction mixture in each reaction. (To do this, first determine the total volume of the solution. Then calculate the mass of the solution, based on the assumption that the added solid does not change the volume and that the density of the solution is the same as that of pure water, 1.0 g/mL.) Remember to add the mass of the solid to the mass of solution in Reactions 1 & 2. Record your results in the calculations table. 3. Calculate the total heat released in each reaction, in J, assuming that the specific heat capacity of the solution is the same as that of pure water, 4.184 J/g°C. Record the result in the calculations table. (Remember: heat of reaction = m x C x ΔT) 4. Calculate the number of moles of NaOH used in reactions 1 and 2 where n = m/MW. Record the results in the calculations table. 5. Calculate the number of moles of NaOH used in reaction 3 by multiplying the volume of NaOH times the molarity (1.000 mol/L). Record the results in the calculations table. 6. Calculate the enthalpy change, in kJ/mol, of NaOH for each reaction and record the results in the calculations table. (Show calculations for each reaction in the spaces below or attach additional pages; Round answers to 3 sig figs) Reaction 1 Reaction 2 Reaction 3 Questions: 1. Show that the equations for reactions 1 and 3 add up to equal the equation for reaction 2. Include the energy released per mole of NaOH in each equation. Use this information to discuss Hess’s Law as it relates to this experiment. 2. Calculate the percent difference between the heat given off in reaction 2 and the sum of the heats given off in reactions 1 and 3. Assume that the heat given off in reaction 2 is correct. % Difference = H 2 -( H1 + H3 x100 = H2 3. Answer the following questions in regards to part 3 of this experiment, by choosing the appropriate response: a) How would your values for heat of the reaction change if you used 200.0 mL of each reagent instead of 100.0 mL? Increases Decreases Does not change b) How would your values for enthalpy of the reaction change if you used 200.0 mL of each reagent instead of 100.0 mL. Increases Decreases Does not change Be sure to save a copy of your eLab Notebook and submit with your report form when completed.



