Enthalpy Practice, Chemistry 161 Name______________________________
advertisement

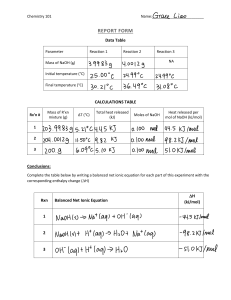
Enthalpy Practice, Chemistry 161 Name______________________________ 1. A reaction occurs between 30.0mL of 1.000M phosphoric acid (H3PO4) and 45.0mL of 1.000M sodium hydroxide (NaOH) base to form water and sodium phosphate. The temperature of the mixture increases from 25.00C to 28.50C as the reaction proceeds to completion. s(H2O) = 4.184 J/gC density of solution = 1.00 g/mL q=cmT a. Write the balanced equation for the reaction. b. Where would you put the word ‘heat’ in your reaction above, as a reactant or as a product? Is the reaction exothermic or endothermic? c. How much heat (q) was produced by the reaction? d. Calculate H in kJ/mol acid. e. Calculate H in kJ/mol base. f. What is Hrxn, the Enthalpy of the reaction? g. If 56.2 mL of 1.00M phosphoric acid were mixed with 59.6 mL of 1.00M NaOH, how much heat would be released or gained?