worksheet side 2
advertisement

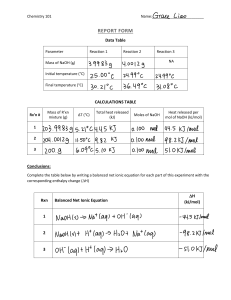
Name __________________ Period ________________ Comparing Heats of Solution for 2 different Ionic Compounds To do this activity: go to: http://www.chem.iastate.edu/group/Greenbowe/sections/projectfolder/flashfiles/thermochem/heat_soln.html Read the Instructions, Part A 1. Select 5.0 grams of NH4Cl and 100.0 ml of water. Use the volume of water to determine the amount of heat released or absorbed in the process of solvating the solute, i.e. calculate Q. 2. Select start, observe temperature change. T1 = ________, T2 = _________ T = _________ 3. Calculate the Q for the process for making a solution of NH4Cl. Use 4.18 J/g C for your calculations. Show calculations for your Q in kJ: Include sign in the Answer Q NH4Cl= ___________________________________kJ 4. This answer is per 5.0 gram. Now convert grams to moles and solve for Q in KJ/ mole (i.e. divide by moles). Compare your answer with the one published in the table 13-5 on p. 410 in your book. Include sign in the Answer Q NH4Cl= _______________kJ/mol. Book value for NH4Cl ____________kJ/mol 5. In your experiment, identify the system ______________ and the surroundings _________________ 6. In your experiment, what gains heat? _________________________What loses heat? ___________________ 7. This process is exothermic or endothermic? Absorbs or releases energy to/from the system/surroundings? Circle correct answers. Part B 1. Select 5.0 grams of NaOH and 100.0 ml of water. Use the volume of water to determine the amount of heat released or absorbed in the process of solvating the solute, i.e. calculate Q. 2. Select start, observe temperature change. T1 = ________, T2 = _________ T = _________ 3. Calculate the Q for the process for making a solution of NaOH. Use 4.18 J/g C for your calculations. Show calculations for your Q in KJ/g: Include sign in the Answer Q NaOH= ____________________ kJ 4. This answer is per 5.0 gram. Now convert grams to moles and solve for Q in KJ/ (i.e. divide by moles). Compare your answer with the one published in the table 13-5 on p. 410 in your book. Include sign in the Answer Q NaOH= _________________ kJ/mol. Book value for NaOH______________ kJ/mol 5. In your experiment, identify the system ______________ and the surroundings _________________. 6. In your experiment, what gains heat? _________________________ What loses heat? ___________________ 7. This process is exothermic or endothermic? Absorbs or releases energy to/from the system/surroundings? Circle correct answers.