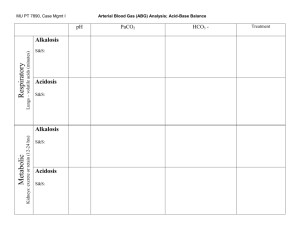
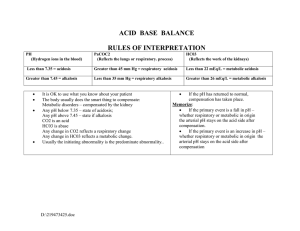
ACID-BASE BALANCE AND IMBALANCE 1 pH 2 Types of Acids: Volatile Acid • Volatile acids: can be converted into gas & eliminated by lungs • Primary volatile acid produced in body = CO2 • @ tissue • • • • Metabolically generated Diffuses into blood Largely travels as HCO3- in blood @ lungs • • HCO3- releases CO2 Blow off CO2 Types of Acids: Fixed Acids • Fixed acids: generated by physiological & pathophysiological mechanisms, eliminated from the body by urinary via kidneys • Catabolism of proteins & phospholipids generates sulfuric acid & phosphoric acid, respectively • Vigorous exercise generates lactic acid • Path ex: diabetic ketoacidosis generates beta-hydroxybutyric acid & acetoacetic acid • Overproduction of fixed acids causes metabolic acids Control of Acids • Body produces more acids than bases • Acid-base homeostasis works to maintain balance between acids & bases Described as body pH • Outside acceptable pH range, proteins are denatured, enzymes lose functionality, & may result in death • 5 The Body and pH • pH range compatible w/ life = 6.8 to 8.0 • Intracellular pH (7.2) is lower than extracellular (7.4) due to transporters in cell membranes • • Na-H pumps move H out of cells to alkalinize intracellular fluids Cl-HCO3- exchange moves HCO3- out of cells to acidify intracellular fluids 6 Changes in pH • Normal arterial pH range = 7.35 to 7.45 • Acidemia = pH < 7.35 • Alkalemia = pH > 7.45 8 Acid-Base Basics • Acids dissociate into H+ • Results in lower pH values • Bases dissociate into OH• Results in higher pH values • Buffers absorb excess ions to maintain pH Arterial Blood Gases: Normal Values • pH: 7.35-7.45 • Partial pressure of oxygen (PaO2): 75 to 100 mmHg • Partial pressure of carbon dioxide (PaCO2): 35-45 mmHg • Bicarbonate (HCO3): 22-26 mEq/L • Oxygen saturation (O2 Sat): 94-100% Regulatory Systems • Chemical buffers Bicarbonate • Phosphate • Protein • • Respiratory system (Lungs) • • Lungs regulate CO2 in blood Renal system Produces bicarbonate • Absorbs acids & bases as needed • Rates of correction • Fastest to slowest • Chemical buffers • • Respiratory • • Responds in sec to min Respond in mins to hours Renal • • Responds in hours to days Do have strongest response 12 Chemical Buffers • Three chemical buffering systems in the body Bicarbonate buffering system in extracellular fluid* • Phosphate intracellular & urinary buffer • Protein intracellular buffer • Bicarbonate buffer • When acidic substance enters blood, HCO3- neutralizes by forming carbonic acid & water • • Prevents blood from becoming too acidic When basic substances enters blood, carbonic acid reacts w/ H+ by forming HCO3- & water • Prevents blood from becoming too basic 14 Protein Buffers • Positively charged amino groups & negatively charges carboxyl groups of amino acids bind H & hydroxyl ions to function as buffers • Nearly all proteins can act as buffers • • Accounts for most buffering w/in cells & a middling amount of buffering power in blood Hemoglobin as a buffer Main protein in RBCs • During conversion of CO2 to HCO3-, H+ are freed • H+ accepted by hemoglobin, by dissociation of Oxygen • • Process revered in lungs to blow off CO2 15 Acidosis • Process that causes increased acidity in blood & tissues • pH <7.35 • Metabolic acidosis results from increased production of metabolic acids or failure to excrete acid in renal system • • Compensated for in lungs Respiratory acidosis results from build up of CO2 in blood due to hypoventilation • • A results of pulmonary dysfunction, head trauma, drugs, etc. Can occur as a compensatory response to chronic metabolic alkalosis Alkalosis • Process that reduces acid (H+) in arterial blood • pH > 7.45 • Respiratory alkalosis results in loss of CO2 • • • Hyperventilation Compensation is releasing H from tissue buffers & excreting HCO3- (renal) Metabolic alkalosis results in loss of H+ or a gain in HCO3• • Vomiting, diarrhea, diuretics Compensation is hypoventilation, increase CO2 18 COMPENSATORY MECHANISMS Respiratory and Renal 19 20 Compensatory Mechanisms • Compensation is the body’s way of restoring a normal blood pH • Compensation DOES NOT treat the root of the problem – the reason for the acidbase imbalance is STILL THERE!!! Respiratory Compensation • Used to compensate for metabolic imbalances only • Chemoreceptors respond to changes in [H+] alters respiratory rate and depth • • Exhalation of carbon dioxide Body pH can be adjusted by changing rate and depth of breathing • • Slower breathing (hypoventilation) hold on to CO2 (↑ PaCO2) and lower pH Faster breathing (hyperventilation) blow off CO2 (↓ PaCO2) and raise pH Respiratory Compensation • Respiratory Rate will… • Increase when blood [H+] is increased • • • • acidic pH/lower pH CO2 is “blown off” Amount of acid in blood is decreased Decrease when [H+] is decreased • • • alkaline pH/higher pH CO2 is retained Amount of acid in blood is increased Respiratory Compensation • This means • Metabolic acidosis causes an increase in rate and depth of ventilation as the body attempts to get rid of acid (CO2) • Metabolic alkalosis causes a decrease in rate and depth of ventilation as the body attempts to retain acid (CO2) Renal Role in (normal) Acid-Base Balance • Reabsorption of filtered HCO3• Proximal tubule • Excretion of excess H+ Buffered by phosphate • As NH4+ • Renal Compensation • Used to compensate for respiratory imbalances • Remember: HCO3- is a base • Kidneys respond to changes in blood pH Excrete H+ and retain HCO3- when acidemia is present • Retain H+ and excrete HCO3- when alkalemia is present • 26 Renal Compensation • This means • A respiratory acidosis will make the kidneys excrete acid (H+) and retain base (HCO3-) • A respiratory alkalosis will make the kidneys excrete base (HCO3-) and retain acid (H+) Renal Compensation • This is the slowest, but most effective regulator of pH • If kidneys fail, pH balance fails • May take hours to days • Ineffective in patients with renal failure Compensated Imbalance • The body is correcting the imbalance • Blood pH is normal • Other blood gas values remain abnormal until the root cause is treated and corrected Uncompensated Imbalance • pH abnormal • Either PaCO2 OR HCO3- abnormal • All other values normal ACID-BASE IMBALANCES And Their Compensations 31 Acid-Base Imbalances • pH < 7.35 acidosis • pH > 7.45 alkalosis • Metabolic or Respiratory • The body’s response to acid-base imbalance is called compensation 32 Respiratory Imbalances • Primary disorders of CO2 (disorders of respiration) Respiratory acidosis: increase in CO2 decrease in pH • Respiratory alkalosis: decrease in CO2 increase in pH • Metabolic Imbalances • Primary disorders involving HCO3- Acidosis: decrease in [HCO3-] decrease in pH • Alkalosis: increase in [HCO3-] increase in pH • • Metabolic mechanisms involve Renal function alteration • Production of acidic metabolic products • Loss of acid from the body • RESPIRATORY IMBALANCES Respiratory Acidosis, Respiratory Alkalosis and their Compensations 35 Uncompensated Respiratory Acidosis • pH < 7.35 • PaCO2 >45 (due to CO2 retention) • HCO3-: WNL 36 Respiratory Acidosis • Any compromise in breathing can result in respiratory acidosis • Hypoventilation CO2 retention decrease in pH • Can result from neuromuscular dysfunction, depression of the brain’s respiratory center, lung disease or airway obstruction Respiratory Acidosis • Acute conditions: Adult Respiratory Distress Syndrome • Pulmonary edema • Pneumothorax • • Chronic conditions: Depression of respiratory center in brain that controls breathing rate – drugs or head trauma • Paralysis of respiratory or chest muscles • Emphysema • 38 Compensation • Kidneys eliminate H+ and retain HCO3- 39 40 ABGs • Uncompensated pH < 7.35 • PaCO2 >45 • HCO3 Normal • • Compensated pH Normal • PaCO2 >45 • HCO3 > 26 (increased; retaining the base to reestablish the acid base balance) • Uncompensated Respiratory Alkalosis • pH > 7.45 • PaCO2 < 35 (loss of CO2) • HCO3: Normal 42 Respiratory Alkalosis • Most common acid-base imbalance • Most commonly results from hyperventilation caused by overstimulation of the respiratory center: pain, hypermetabolic states or acute hypoxia • Other conditions that stimulate respiratory center: Oxygen deficiency at high altitudes • Pulmonary disease • Congestive heart failure • Acute anxiety • Fever, anemia • Cirrhosis • Sepsis • Compensation • Kidneys conserve H+ and excrete HCO3- 44 45 ABG Results • Uncompensated pH > 7.45 • PaCO2 < 35 • HCO3 Normal • • Compensated pH Normal • PaCO2 < 35 • HCO3 < 22 (decreased; excreting the base to reestablish the balance) • Summary: UNCOMP. Resp. Imbalances • Uncompensated respiratory alkalosis • Uncompensated respiratory acidosis • pH > 7.45 • pH < 7.35 • PaCO2 < 35 • PaCO2 > 45 • HCO3- WNL • HCO3- WNL Summary: COMPENSATED Resp. Imbalances • Compensated Respiratory Alkalosis • Compensated Respiratory Acidosis • pH = WNL but closer to 7.45 • pH = WNL but closer to 7.35 • PaCO2 < 35 • PaCO2 > 45 • HCO3- < 22 • HCO3- > 26 METABOLIC IMBALANCES Metabolic Acidosis, Metabolic Alkalosis and their Compensations 49 Uncompensated Metabolic Acidosis • pH < 7.35 • PaCO2: Normal • HCO3 < 22 (direct or relative; loss of HCO3- or gain of H+) 50 Metabolic Acidosis • Characterized by gain of acid or loss of bicarb Loss of bicarbonate through diarrhea or renal dysfunction • Gain of acid • • • Production of ketone bodies as with diabetes mellitus, alcoholism, starvation, & hyperthyroidism Failure of kidneys to excrete H+ 51 Compensation • Increased ventilation • Renal excretion of hydrogen 52 53 ABG Results • Uncompensated pH < 7.35 • PaCO2 Normal • HCO3 < 22 • • Compensated pH Normal • PaCO2 < 35 (decreased: loss of acid/CO2 to reestablish the balance) • HCO3 < 22 • Uncompensated Metabolic Alkalosis • pH > 7.45 • PaCO2: Normal • HCO3 >26 (direct or relative: increase in HCO3- or loss of H+) 55 Metabolic Alkalosis • Causes: Excess vomiting = loss of stomach acid • Excessive use of alkaline drugs • Certain diuretics • Endocrine disorders • Heavy ingestion of antacids • Severe dehydration • Compensation • Respiratory compensation difficult – hypoventilation limited by hypoxia 57 58 ABG Results • Uncompensated pH > 7.45 • PaCO2 Normal • HCO3 >26 • • Compensated pH Normal • PaCO2 > 45 (increased; retaining CO2/acid to reestablish the balance) • HCO3 > 26 • Summary: UNCOMP. Metabolic Imbalances. • Uncompensated metabolic alkalosis • Uncompensated metabolic acidosis • pH > 7.45 • pH < 7.35 • PaCO2 = WNL • PaCO2 = WNL • HCO3- > 26 • HCO3- < 22 Summary: COMP. Metabolic Imbalances • Compensated Metabolic Alkalosis • Compensated Metabolic Acidosis • pH = WNL but closer to 7.45 • pH = WNL but closer to 7.35 • PaCO2 > 45 • PaCO2 < 35 • HCO3- > 26 • HCO3- < 22 INTERPRETING ABGS Normal ABG • pH and PaCO2 move in opposite directions As PaCO2 increases, pH decreases • As PaCO2 decreases, pH increases • • HCO3- and PaCO2 move in the same direction H2O + CO2 H2CO3 H+ + HCO3• As PaCO2 increases, HCO3- increases • As PaCO2 decreases, HCO3- decreases • 63 If pH is ABNORMAL… • If pH and PaCO2 move in the SAME direction, the primary problem is METABOLIC • • • Increased pH and increased PaCO2 = Metabolic alkalosis Decreased pH and decreased PaCO2 = Metabolic acidosis If pH and PaCO2 move in the OPPOSITE direction, the problem is RESPIRATORY • • Increased pH and decreased PaCO2 = Respiratory alkalosis Decreased pH and increased PaCO2 = Respiratory acidosis 64 Mixed Disorders ARE Possible • If HCO3- and PaCO2 change in opposite directions (which they normally should not) then it’s a mixed disorder • We won’t get into this scenario 65 Identify the Primary Disorder: Look at pH • < 7.35 is acidosis • > 7.45 is alkalosis http://www.rnceus.com/abgs/abgmethod.html Look at the PaCO2 • If it changes in the SAME direction as the pH, the primary disorder is METABOLIC • • • If it is high (>45) it is METABOLIC alkalosis If it is low (< 35) it is METABOLIC acidosis If it changes in the OPPOSITE direction as the pH, the primary disorder is RESPIRATORY 67 68 http://www.thoracic.org/clinical/critical-care/clinical-education/abgs.php Look for Compensation 69 http://www.thoracic.org/clinical/critical-care/clinical-education/abgs.php Example • A patient is in intensive care because he suffered a severe myocardial infarction 3 days ago. The lab reports the following values from an arterial blood sample: pH 7.3 • HCO3- = 20 mEq / L ( 22 - 26) • PaCO2 = 32 mm Hg (35 - 45) • 70 Check the pH • pH = 7.3 • acidosis What is the PaCO2? • PaCO2 = 32 mm Hg (35 - 45) • • Low PaCO2 Move is in the same direction as pH • • Low pH, low PaCO2 Metabolic Look at the HCO3• HCO3- = 20 mEq / L ( 22 - 26) • • Low Means there must be a compensation going on Look for Compensation • If a change is seen in BOTH PaCO2 and HCO3-, the body is trying to compensate • • HCO3- = 20 mEq / L ( 22 - 26) PaCO2 = 32 mm Hg (35 - 45) Diagnosis • Metabolic acidosis • With compensation 75