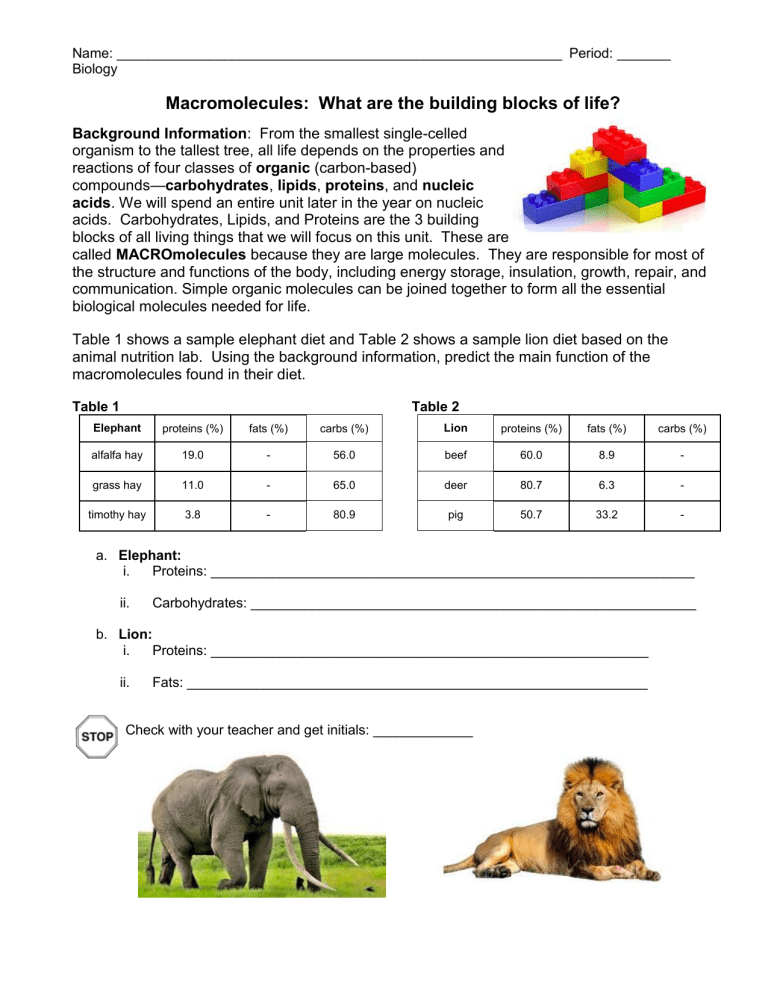
Name: __________________________________________________________ Period: _______ Biology Macromolecules: What are the building blocks of life? Background Information: From the smallest single-celled organism to the tallest tree, all life depends on the properties and reactions of four classes of organic (carbon-based) compounds—carbohydrates, lipids, proteins, and nucleic acids. We will spend an entire unit later in the year on nucleic acids. Carbohydrates, Lipids, and Proteins are the 3 building blocks of all living things that we will focus on this unit. These are called MACROmolecules because they are large molecules. They are responsible for most of the structure and functions of the body, including energy storage, insulation, growth, repair, and communication. Simple organic molecules can be joined together to form all the essential biological molecules needed for life. Table 1 shows a sample elephant diet and Table 2 shows a sample lion diet based on the animal nutrition lab. Using the background information, predict the main function of the macromolecules found in their diet. Table 1 Table 2 Elephant proteins (%) fats (%) carbs (%) Lion proteins (%) fats (%) carbs (%) alfalfa hay 19.0 - 56.0 beef 60.0 8.9 - grass hay 11.0 - 65.0 deer 80.7 6.3 - timothy hay 3.8 - 80.9 pig 50.7 33.2 - a. Elephant: i. Proteins: _______________________________________________________________ ii. Carbohydrates: __________________________________________________________ b. Lion: i. Proteins: _________________________________________________________ ii. Fats: ____________________________________________________________ Check with your teacher and get initials: _____________ Directions: Analyze Model 1 and answer the questions that follow. MODEL 1: Macromolecules of Life are made from repeating smaller monomer units such as glucose, glycerol and glycine. Carbohydrate Monosaccharide (glucose) Lipid Lipid (fatty acid) Protein Amino Acid (glycine) Key: H = Hydrogen; O = Oxygen; C = Carbon; N = Nitrogen Questions: 1. A complete carbohydrate is a macromolecule. This means that it is a large or small molecule. (circle one) 2. Use Model 1 to show which atoms are present in each type of molecule by listing the symbol for each atom included (an atom = element in the molecule). Carbohydrate has been done for you. a. Carbohydrate: C, H, O b. Lipid: ______________________ c. Protein: ________________________ 3. List 3 similarities between the 3 types of macromolecules. a. _________________________________________________________ b. _________________________________________________________ c. _________________________________________________________ 4. List 3 differences between the 3 types of macromolecules. a. _________________________________________________________ b. _________________________________________________________ c. _________________________________________________________ READ THIS! During chemical reactions, the bonds in molecules are continually broken and reformed. When bonds are broken, energy is released called ATP. ATP molecules are energy molecules that allow cells to do the work needed to keep the organism alive and functioning. When bonds are formed, energy is absorbed. If more energy is released than absorbed during a chemical change, the process can be used as a source of energy. A general rule for processes such as respiration is the more carbon atoms there are in a molecule, the more energy that molecule can provide to the organism when it is used as food. 5. Using the information from above and Model 1, is a carbohydrate, lipid, or a protein more likely to be a good source of energy for an organism? Use comparative data to support your answer. _______________________________________________________________________________ _______________________________________________________________________________ Check with your teacher and get initials: _____________ Background Information: There are many chemical reactions occurring in your body right now. As you eat food, these food molecules are broken down by enzymes and a chemical reaction takes place. The bonds between elements are broken and reformed – energy can be given off or taken in when the reaction occurs. The left side (before arrow) of the reaction (what goes in) is called the reactants while the right side (after arrow) of the reaction (what comes out) is called the products. The number of elements that go into the reaction should be the same number of elements that come out of the reaction. Analyze the two models showing the elephant’s diet and lion’s diet and answer the questions that follow. MODEL 2: Elephant’s Diet - Carbohydrates 6. The reactants are ________________________ and ____________________________ 7. The products are _________________________ and ____________________________ 8. Would you say that glucose or sucrose is more complex? (circle one) Why? _____________________________________________________________________________ _____________________________________________________________________________ 9. What would you call the process that is occurring? ___________________________________ 10. What is the elephant getting when the bond is broken in sucrose? _______________________ 11. Starch is a complex carbohydrate found in plants which contains approximately 200 glucose molecules. Predict the function of starch and explain why. ______________________________ _____________________________________________________________________________ Check with your teacher and get initials: _____________ MODEL 3: Elephant’s Diet and Lion’s Diet - Proteins 12. What would you call the process that is occurring? _________________________________ Read This! There are only 20 different amino acids found in the body, however, there are thousands of different proteins being used by your cells. Proteins differ in their types and sequence of the number of amino acids which allows for the thousands of different proteins found in your body. When you consume protein, it is broken down into individual amino acids. These amino acids are then used to make new proteins. Amino acid 1 Amino acid 2 Amino acid 3 13. Based on the information above about how proteins are made and are different, bond the 3 amino acids above together to create a protein. Remember that each carbon can only form 4 bonds. Circle the OH from one amino acid with the H of the next amino acid (to become H2O) that must drop off to bond the carbon at the end of one amino acid to the nitrogen of the next. Use pencil! Check with your teacher and get initials: _____________ MODEL 4: Lion’s Diet - Lipids 14. The reactants are __________________________ and ___________________________ 15. The products are ___________________________ and ___________________________ 16. Because a monoglyceride molecule has more carbon atoms than a glucose molecule, you can assume that … ________________________________________________________________________________ ________________________________________________________________________________ Check with your teacher and get initials: _____________ Name: __________________________________________________________ Period: _______ BREAKING IT ALL DOWN… LITERALLY! Simple context statement: In order for cells (plant or animal) to create ATP energy molecules that allow the cells to do the important work of keeping an organism alive, they need to further break down the macromolecules either made in plant cells through photosynthesis or break down the macromolecules in the foods they eat. ATP C-C ATP C-C Q1: Does the above statement make sense to you? (Talk with your group and check the box below that best fits your thinking.) C-C _____ YES _____ NO Q2: If ATP energy molecules help cells do work, for which of the following processes do they support? (Circle all that apply) Cells growing Cells moving ATP Cells dividing/reproducing Cells metabolizing food molecules Cells absorbing substances from their environment Cells expelling waste Q3: Below are the three monomers (building blocks) again. Observe the cell model above. What does each molecule below have in common that the cell needs? __________________________ Carbohydrate Monosaccharide (glucose) Lipid Lipid (fatty acid) Protein Amino Acid (glycine) Q4: Look at Figure 1 on your table. Count each bond between the carbons in the molecules above and multiple that by the energy produced when broken. Draw a slash (/) between each carbon. Carbohydrate: _______ Lipid: _______ Protein: _______ Q5: Based on your answer to Q3, how many of those units does each of the above have? Circle each “unit” in the above molecules. Carbohydrate: _______ Lipid: _______ Protein: _______ Q6: Based on your calculations, which of the 3 macromolecules directly above has the most potential energy (kilocalories) per molecule? _____________________________________________________ Q7: Make sense as to why this macromolecule is used by organisms in the way it is based on your answer to the last question. ____________________________________________________________ ___________________________________________________________________________________ Biology - Macromolecule CER Name: ________________________ Claim: Elephants and Lions need energy to survive. Evidence: Use Tables 1 and 2 (on page 1 of “Macromolecules: What are the building blocks of life”) to provide data. Elephants use mostly ___________________________ for energy. ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ Lions use mostly ___________________________ for energy. ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ Reasoning: Use the Read This! (on page 2 of “Macromolecules: What are the building blocks of life”) and Models 2, 3, and 4 to support your reasoning. Elephants ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ Lions ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ Figure 1. The ENERGY CHALLENGE! Name: _________________________________ Using the carbohydrate glucose from Q3 and Figure 1 at your table, add up the potential energy available in that molecule in all bonds. Do your calculation in the box at the left. Now using the lipid (fatty acid) from Q3 and Figure 1 at your table, add up the potential energy available in that molecule if all bonds. Do your calculation in the box at the right. Carbohydrate TOTAL ENERGY: ______________________________ Lipid TOTAL ENERGY: ______________________________