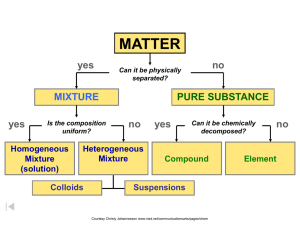
Classification of Matter Use to make foldable The terms in red are your voc. terms. Pure Substances • Pure Substance that cannot be broken down into any other substances by chemical or physical means Gold - element Manganese Dioxide - compound Pure Substance • Element – composed of identical atoms – EX: copper wire, aluminum foil Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Pure Substances • Compound – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – Chemical bonds hold the elements together – EX: table salt (NaCl) Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Pure Substances - FYI Law of Definite Composition – A given compound always contains the same, fixed ratio of elements. Two different compounds, each has a definite composition Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Molecules • Groups of two or more atoms bound by chemical bonds • Can be two of the same element Chemical Formula-Extra Info • Shows the compound and the ratio of atoms Diatomic Elements, 1 and 7 H2 N2 O2 F2 Cl2 Br2 F2 Matter Flowchart Examples: – graphite element – pepper hetero. mixture – sugar (sucrose) compound – paint hetero. mixture – soda solution homo. mixture Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Classification of Matter uniform properties? fixed composition? no heterogeneous mixture no solution no element yes compound chemically decomposable? http://antoine.frostburg.edu/chem/senese/101/matter/slides/sld003.htm Both elements and compounds have a definite makeup and definite properties. Elements only one kind of atom; atoms are bonded it the element is diatomic or polyatomic substance with definite makeup and properties Packard, Jacobs, Marshall, Chemistry Pearson AGS Globe, page (Figure 2.4.1) Compounds two or more kinds of atoms that are bonded Mixtures two or more kinds of and two or more substances that are physically mixed Mixtures Variable combination of two or more pure substances. Each keep individual properties Heterogeneous – Can see different parts (different) Homogeneous- Evenly Mixed cannot see different parts. (Same) Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Tyndall Effect • The scattering of light by particles in a mixture • http://www.youtube.com/watch?v=gheuYq Q6phE&feature=related Mixtures Solution – homogeneous – very small particles – no Tyndall effect – particles don’t settle – EX: – rubbing alcohol (ethyl alcohol and water) – Air (nitrogen and oxygen) Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Mixtures Colloid – heterogeneous – medium-sized particles – Tyndall effect – particles don’t settle – Particles scatter light – EX: • • • • Milk Clouds Smoke mayo Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Mixtures Suspension – heterogeneous – large particles – Tyndall effect – particles settle – EX: • fresh-squeezed lemonade • Sand in water Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Mixtures Examples: – mayonnaise colloid – muddy water suspension – fog colloid – saltwater solution – Italian salad dressing suspension Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Elements, Compounds, and Mixtures hydrogen atoms oxygen atoms (a) an element (hydrogen) (b) a compound (water) hydrogen atoms Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 68 (c) a mixture (hydrogen and oxygen) (d) a mixture (hydrogen and oxygen) MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem no Element Classification of Matter MATTER (gas. Liquid, solid, plasma) Separated by PURE SUBSTANCES MIXTURES physical means into Separated by COMPOUNDS ELEMENTS chemical means into Kotz & Treichel, Chemistry & Chemical Reactivity, 3rd Edition , 1996, page 31 HOMOGENEOUS MIXTURES HETEROGENEOUS MIXTURE Classification of Matter Matter Physically separable Substance Definite composition (homogeneous) Element (Examples: iron, sulfur, carbon, hydrogen, oxygen, silver) Chemically separable Mixture of Substances Variable composition Compound (Examples: water. iron (II) sulfide, methane, Aluminum silicate) Homogeneous mixture Heterogeneous mixture Uniform throughout, also called a solution (Examples: air, tap water, gold alloy) Nonuniform distinct phases (Examples: soup, concrete, granite) Mixture vs. Compound Different Alike Variable Composition Involve substances Topic No bonds between components Can be separated by physical means Mixture Different Fixed Composition Topic Contain two or more elements Can be separated into elements Compound Bonds between components Can ONLY be separated by chemical means Compounds vs. Mixtures • Compounds have properties that are uniquely different from the elements from which they are made. – A formula can always be written for a compound – e.g. NaCl Na + Cl2 • Mixtures retain their individual properties. – e.g. Salt water is salty and wet Top Ten Elements in the Universe Element 1. Hydrogen 2. Helium 3. Oxygen 4. Carbon 5. Neon 6. Iron 7. Nitrogen 8. Silicon 9. Magnesium 10. Sulfur Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 26 Percent (by atoms) 73.9 24.0 1.1 0.46 0.13 0.11 0.097 0.065 0.058 0.044 A typical spiral galaxy (Milky Way is a spiral galaxy) The Composition of Air Nitrogen Helium Neon Oxygen Water vapor Air Carbon dioxide Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 34 Argon Chart Examining Some Components of Air Nitrogen consists of molecules consisting of two atoms of nitrogen: N2 Oxygen consists of molecules consisting of two atoms of oxygen: O2 Water consists of molecules consisting of two hydrogen atoms and one oxygen atom: H2O Argon consists of individual argon atoms: Ar Carbon dioxide consists of molecules consisting of two oxygen atoms and one carbon atom: CO2 Neon consists of individual neon atoms: Helium consists of individual helium atoms: Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 35 Ne He Reviewing Concepts Classifying Matter • Why does every sample of a given substance have the same properties? • Explain why the composition of an element is fixed. • Describe the composition of a compound. • Why can the properties of a mixture vary? • On what basis can mixtures be classified as solutions, suspensions, or colloids?