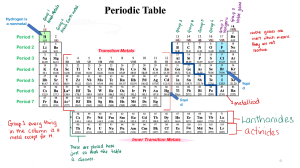
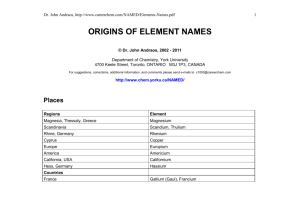
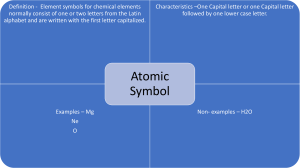
CHEMISTRY 1. Period, Group 2. 91 metals Lithium Indium Bismuth Beryllium Tin Polonium Sodium Cesium Francium Magnesium Barium Radium Aluminum Lanthanum Actinium Potassium Cerium Thorium Calcium Praseodymium Protactinium Scandium Neodymium Uranium Titanium Promethium Neptunium Vanadium Samarium Plutonium Chromium Europium Americium Manganese Gadolinium Curium Iron Terbium Berkelium Cobalt Dysprosium Californium Nickel Holmium Einsteinium Copper Erbium Fermium Zinc Thulium Mendelevium Gallium Ytterbium Nobelium Rubidium Lutetium Lawrencium Strontium Hafnium Rutherfordium Yttrium Tantalum Dubnium Zirconium Tungsten Seaborgium Niobium Rhenium Bohrium Molybdenum Osmium Hassium Technetium Iridium Meitnerium Ruthenium Platinum Darmstadtium Rhodium Gold Roentgenium Palladium Mercury Copernicium Silver Thallium Ununtrium Cadmium Lead Flevorium Livermorium 3. Lithium, Beryllium, Boron, Carbon, Sodium, Magnesium, Aluminum, Silicon, Phosphorus, Sulfur, Potassium, Calcium, Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Gallium, Germanium, Arsenic, Selenium, Rubidium, Strontium, Yttrium, Zirconium, Niobium, Molybdenum, Technetium, Ruthenium, Rhodium, Palladium, Silver, Cadmium, Indium, Tin, Antimony, Tellurium, Iodine, Cesium, Barium, Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Hafnium, Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Thallium, Lead, Bismuth, Polonium, Astatine, Francium, Radium, Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Neutrons, Tetraneutron 3. GAS AT ROOM TEMPERATURE: Hydrogen, Helium, Nitrogen, Oxygen, Flourine, Neon, Chlorine, Argon, Krypton, Xenon, Radon 4. The period 6 inner transition metals (lanthanides) are cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). The period 7 inner transition metals (actinides) are thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), americium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No), and lawrencium (Lr). 5. The elements found as diatomic molecules are hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine and iodine (Their molecular formulas would be written as H2, N2, O2, F2, Cl2, Br2, and I2.) 6. List of Representative Elements in S Block The S Block elements or the elements in columns 1A and 2A on the left of the periodic table include Hydrogen (H), Lithium (Li), Sodium (Na), Potassium (K). Rubidium (Rb), Cesium (Cs), Francium (Fr), Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). List of Representative Elements in P Block The P Block elements or the elements in columns 3A through 8A on the right of the periodic table include: Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl), Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb), Ununquadium (Uuq), Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi), Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), Polonium (Po), Fluoride (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At), Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn) 7. Argon is not a metal it is a noble gas. The name argon comes from the Greek word argos, "the lazy one." The name is based on argon's inability to react with anything. Argon is chemically inactive. On rare occasions, and under extreme conditions, it forms weak, compound-like structures. 8. Chromium (Cr), Molybdenum (Mo), Tungsten (W), and Seaborgium (Sg) are all transition metals they are refractory metals. This metals are highly resistant to heat and wear. 9. The most reactive nonmetal is fluorine. Fluorine is a halogen, which is Group 17 on the periodic table. 10. A group of three malleable ductile transition metals forming group 11 (formerly IB) of the periodic table: copper (Cu), silver (Ag), and gold (Au). GIVE/RECEIVE Element 1. Calcium 2. Lithium 3. Iodine 4. Oxygen 5. Magnesium 6. Sodium 7. Chlorine 8. Potassium 9. Sulfur 10. Fluorine Give/Receive Give Give Receive Receive Give Give Receive Give Receive Receive Metal/Non-Metal Metal Metal Nonmetal Nonmetal Metal Metal Nonmetal Metal Nonmetal Nonmetal NOTE: Jaslene Metals lose electrons While Nonmetals Gain electrons SINGLE/ COUPLE 1. 2. 3. 4. 5. Iron – Single Sodium Chloride – Couple Magnesium Iodide – Couple Mercury – Single Astatine – Single B. 6. Chlorine – Couple 7. Astatine – Single 8. Iodine – Couple 9. Oxygen – Couple 10. Carbon - Single