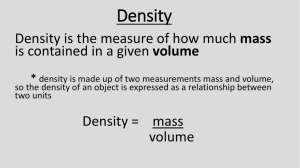Name____________________Date________________Hour 1 2 3 4 5 6 7 Score______CB___ ___ Density Practice Problems For each problem, calculate the density. Make sure you include the SI units with each answer. Be sure to show all of your work in the space provided. Remember: density = mass / volume Getting Started 1. Write out the mathematical equation for density. 2. Which formula is correctly used to measure for density? A. Width x length x height B. Mass divided by volume Level 1 1. An object has a mass of 4 grams and an volume of 2 cm3. What is the density of the object? 𝐷= 𝑀 𝑉 2. An object has a mass of 10 grams and a volume of 5 cm3. What is the density of the object? 𝐷= 𝑀 𝑉 3. A rock has a mass of 15 grams and a volume of 5 cm3. What is the density of the rock? 𝐷= 𝑀 𝑉 Level 2 4. An object has a volume of 5 cm3 and a mass of 20 g. What is the density of the object? 5. An object has a volume of 2 cm3 and a mass of 4 g. What is the density of the object? 6. A rock has a volume of 6 cm3 and a mass of 24g. What is the density of the rock? 1 Level 3 7. A pumpkin has a volume of 10 cm3 and a mass of 8 g. What is the density of the pumpkin? 8. What is the density of a rock with a volume of 5 cubic centimeters and a mass of 3 grams? 9. An object has a mass of 20.0 grams and a volume of 5.0 cubic centimeters. Give the density. Level 4 10. An object has a mass of 48 g and it has a height of 5 cm a width of 2 cm and a length of 5 cm. what is the density of the object? 11. An object is a regular, rectangular, solid with dimensions of 2 cm by 3cm by 2cm. It has a mass of 24 g. Find its density. 12. If an object is an irregularly shaped solid and it is dropped into a graduated cylinder and it displaces 25 mL of water and has a mass of 50 grams what is the density of this irregular shaped object? 13. A square chunk of plastic has a length of 5 cm, width of 5 cm and height of 5 cm. It has a mass of 200 g. What is it’s density Level 5 14-18. List the substances A-E in order from most dense to least dense based on the facts provided. Please write your answers in the spaces provided below. Substance A: Substance B: Substance C: Substance D: Substance E: 8.2 g/cm3 3.5 cm3 and 30.0 g 10.0 g and 40 mL 0.5 g/cm3 2.0 cm by 3.0 cm by 1.0 cm and 4.0 grams Most Dense ___ ___ ___ ___ 2 ___ Least Dense Level 6 19-21. Fill in the blanks in the following table Substance X Y Z Volume (cm3) 20.0 Mass (g) 10.0 50.0 Density (g/cm3) 5.0 10.0 4.0 Level 7 22. Water has a density of 1g/mL. There are four rocks that all have the same volume of 10 cubic centimeters. The mass for each of these rocks is given below. Which one of the four rocks will float when placed in water? A. 30 grams B. 20 grams C. 12 grams D. 8 grams Level 8 23. An irregularly shaped object displaces 32 ml of water. It has a mass of 9.0 grams. 24. A rock dropped in a graduated cylinder raises the level of water from 20 mL to 35 mL. The rock has a mass of 45 g. What is the density of the rock? 25. Water has a density of 1.0 g/mL. You are given a rock with a density of 2.5 g/mL. Predict what will happen to the rock when put into a container of water. 3 Level 9 26. A liquid has a density of 1g/mL. If you have 50mL of the liquid, what would its mass be? 27. A liquid is found to have a volume of 50 mL (in a graduated cylinder). When placed on a balance, the liquid and graduated cylinder has a mass of 125 g. The empty graduated cylinder has a mass of 75 g. What is the density of the liquid? 28. A student has two objects. Object 1 has a mass of 10 g and a volume of 5 cubic centimeters. Object 2 has a mass of 10 g and a volume of 20 cubic centimeters. If both objects are placed in water, which will float and why? Level 10 29. Did you know that an unopened 355 mL can of diet Dr. Pepper will float in a lake? That’s a fact! However, it’s also a fact that an unopened 355mL can of regular Dr. Pepper will sink in that same lake! Based on these two facts, which type of soda is the least dense? Explain your answer. 30. Density can be calculated by dividing the mass of an object by its volume. Water has a density of 1g/ml. If an object’s density is greater than 1 g/ml it will sink and if its density is less than 1g/ml it will float. Object A has a mass of 40g and a volume of 30mL and object B has a mass of 15 g and a volume of 20 mL. What will happen when both objects are placed in water? A. B. C. D. Object A will float because its density is less than water Object B will sink because its density is greater than water Object A will sink because its density is greater than water Objects A and B will both float because their density is the same as water 4