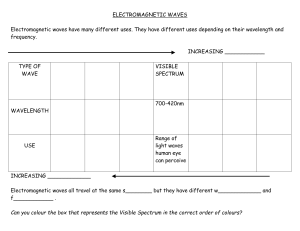
Atomic Spectrum Notes/Practice Problems Part A: Concepts 1. Electromagnetic Spectrum – A range containing all the possible frequencies and wavelengths of electromagnetic radiation 2. The Sun is the main source of the electromagnetic energy we use. 3. Electromagnetic radiation is a type of energy that travels in waves. 4. Electromagnetic waves can be described in terms of its wavelength and frequency. ● All electromagnetic waves travel through space at the same velocity known as the speed of light o c = 3.00 x 108 m/s ● All electromagnetic waves have the same basic form, as shown in the diagram below. The distance between two consecutive peaks of a wave is its wavelength. Every wave has a characteristic wavelength. ● Wavelength is an identifying property of a wave o The Greek symbol λ (lambda) is used to denote wavelength o Wavelength is measured in meters (m) ● Frequency is an identifying property of a wave o Determined by the number of peaks that pass through a fixed point every second o Frequency is expressed in s-1 or Hz o Every wave has a characteristic frequency. High-frequency waves have more energy than low-frequency waves. 5. Wavelength and related. Frequency are inversely ● As wavelength increases, frequency decreases and/or as frequency increases, wavelength decreases ● Relationship between wavelength and frequency represented by this equation: o c=λxf ▪ λ = wavelength (m) ▪ f = frequency (s-1 or Hz) ▪ c = 3.00 x 108 m/s (Speed of Light; constant) 6. Max Planck proposed that electromagnetic radiation was emitted in photons, which are small packets of energy. ● Planck’s constant is equal to 6.626 x 10-34 Js 7. Energy and Frequency are directly related. ● As frequency increases, energy increases ● Represented by the equation: o E = hf ▪ E = energy (J) ▪ h = 6.626 x 10-34 Js (Planck’s constant) ▪ f = frequency (s-1 or Hz) 8. Mathematical Equations for Energy, Frequency, and Wavelength ● c=λxf o Solved for wavelength: ▪ λ=c/f o Solved for frequency: ▪ f=c/λ ● E = hf o Using wavelength to solve for energy ▪ E = (h x c) / λ 9. Electromagnetic Spectrum: Wavelength, Frequency, Energy Part B: Calculations 1. The relationship between wavelength and frequency is c = λ x f. Calculate the wavelength of an electromagnetic wave that has a frequency of 5.2 x 1012 Hz. The speed of light is 3.0x108 m/s. 2. The relationship between wavelength and frequency is c = λ x f. If the wavelength of an electromagnetic wave is 4.8 x 10-12 m, calculate the frequency in Hz. The Speed of Light is 3.0 x 108 m/s. 3. The relationship between energy and frequency is E = h x f. A microwave can have a frequency of 3.8 X 1010 Hz. How much energy does this microwave give off in joules? Planck's Constant is 6.626 x 10-34 Js. 4. The relationship between wavelength and energy is E = (h x c) / λ. A typical cell phone uses wavelengths of 0.36 meters. How much energy does this wave have? Planck's Constant is 6.626 x 10-34 Js. The speed of light is 3.00 x 108 m/s. 5. The relationship between frequency and energy is E = h x f. A particular nuclear process releases a single gamma ray photon with an energy of 4.75x10-14 joules. What is the frequency of this radiation? Planck's Constant is 6.626 x 10-34 Js.