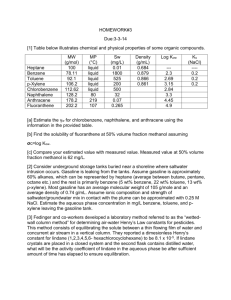
Bioremediation of hydrocarbon from waste water by bacterial culture Thesis submitted for the partial fulfillment of the degree of Bachelor of Technology in Biotechnology by Soham Ray Chaudhuri University Roll number: 12617004041 University Registration number: 171260110470 Under the guidance of Prof (Dr.) Bhaswati Chakraborty Assistant Professor and Prof. Sonali Hazra Das (co-guide) Assistant Professor DEPARTMENT OF BIOTECHNOLOGY HERITAGE INSTITUTE OF TECHNOLOGY, KOLKATA 1 CONTENTS 1. Introduction .....................................................................................................……………………………….2 Hydrocarbons and their chemical structure.................................................................................. 2-4 2. Advantages of hydrocarbons…………...................................................................................................4 3. Industrial sources of hydrocarbons in waste water ........................................................................5-7 4. Effect of hydrocarbons on the environment ......................................................................................8 5. Removal necessity and purification methods…………....................................................................9=13 6. Bioremediation…………………………………………….............................................................................13-14 Role of microorganisms for the removal of hydrocarbons.....................................................14-16 Aerobic and anaerobic degradation of wastewater hydrocarbons.............................................16 Pathways for degradation of aromatic hydrocarbons............................................................17-19 Factors affecting bioremediation ..........................................................................................19-21 7. Bioremediation over conventional processes.............................................................................21=23 8. Permissible limits for hydrocarbons in water .............................................................................23-24 9. Literature review……………………………………...................................................................................25-37 10. Acknowledgement..........................................................................................................................38 11. Bibliography………......................................................................................................................39=42 2 1.Introduction Hydrocarbons and their chemical structure Hydrocarbons are organic compounds that contain only carbon and hydrogen. The inherent ability of hydrocarbons to bond to themselves is known as catenation, and allows hydrocarbon to form more complex molecules, such as cyclohexane and benzene. Catenation comes from the fact that the bond character between carbon atoms is entirely non-polar. The four general classes of hydrocarbons are: alkanes, alkenes, alkynes and arenes. Aromatic compounds derive their names from the fact that many of these compounds in the early days of discovery were grouped because they were oils with fragrant odours. Saturated hydrocarbons (alkanes) are the simplest of the hydrocarbon species. They are composed entirely of single bonds and are saturated with hydrogen. The general formula for saturated hydrocarbons is CnH2n+2 (assuming non-cyclic structures). Saturated hydrocarbons are the basis of petroleum fuels and are found as either linear or branched species. The simplest alkanes have their C atoms bonded in a straight chain; these are called normal alkanes. They are named according to the number of carbon atoms in the chain. The smallest alkane is methane (CH4). Alkanes burn in the presence of oxygen, a highly exothermic oxidationreduction reaction that produces carbon dioxide and water. As a consequence, alkanes are excellent fuels. For example, methane, CH4, is the principal component of natural gas. Butane, C4H10, used in camping stoves and lighters is an alkane. Gasoline is a liquid mixture of continuous- and branched-chain alkanes, each containing from five to nine carbon atoms, plus various additives to improve its performance as a fuel. Kerosene, diesel oil, and fuel oil are primarily mixtures of alkanes with higher molecular masses. The main source of these liquid alkane fuels is crude oil, a complex mixture that is separated by fractional distillation. Fractional distillation takes advantage of differences in the boiling points of the components of the mixture. Unsaturated hydrocarbons have double and/or triple bonds between carbon atoms. Those with double bond are called alkenes and have the general formula CnH2n (assuming non-cyclic structures). Those with triple bonds are called alkynes and have general formula CnH2n-2. The smallest alkene—ethene—has two C atoms and is also known by its common name ethylene and the smallest alkyne is ethyne, also known as acetylene. Alkenes are much more reactive than alkanes because the C=CC=C moiety is a reactive functional group. A π bond, being a weaker bond, is disrupted much more easily than a σ bond. Thus, alkenes undergo a 3 characteristic reaction in which the π bond is broken and replaced by two σ bonds. This reaction is called an addition reaction. The hybridization of the carbon atoms in the double bond in an alkene changes from sp2 to sp3 during an addition reaction. For example, halogens add to the double bond in an alkene instead of replacing hydrogen, as occurs in an alkane. Chemically, the alkynes are similar to the alkenes. Since the C≡CC≡C functional group has two π bonds, alkynes typically react even more readily, and react with twice as much reagent in addition reactions. Cycloalkanes are hydrocarbons containing one or more carbon rings to which hydrogen atoms are attached. The prefix "cyclo" is added to the name to communicate the ring structure. The general formula for a saturated hydrocarbon containing one ring is CnH2n. Aromatic hydrocarbons, also known as arenes, are hydrocarbons that have at least one aromatic ring. Aromatic compounds contain the benzene unit. Benzene itself is composed of six C atoms in a ring, with alternating single and double C–C bonds. The natural sources of hydrocarbons include coal, petroleum, and natural gas. These are often known as fossil fuels because they are the remains of animals and plants which died millions of years ago; their remains have become deposited and transformed into sediment as a result of the great heat and pressure in the earth's crust. They are used as fuels, burnt to release heat and other forms of energy. Coal is a solid fuel, petroleum is a dark and viscous liquid fuel (otherwise called crude oil), and natural gas is a gaseous fuel. Fig 1 4 Naphthalene Naphthalene is a bicyclic aromatic hydrocarbon derived from coal tar or crude oil. It is an insecticide that is also used as a repellent. Its International Union of Pure and Applied Chemistry (IUPAC) name is naphthalene. Naphthalene and other polycyclic aromatic hydrocarbons (PAHs) are released from incomplete combustion processes originating in industry, domestic sources including cigarette smoke and motor vehicle exhaust, as well as natural events such as forest fires. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It has a chemical formula C10H8 and is the simplest polycyclic aromatic hydrocarbon. At NTP, naphthalene is a white crystalline solid. Fig 2: Naphthalene structure 2. Advantages of hydrocarbons Hydrocarbons, as a class, represent compounds with an excellent store of energy. Some idea of the available energy may be obtained by comparing the heat of combustion of some six carbon compounds. For example, glucose yields 674 Calories, as compared to 1,002 Calories from hexane. Knowledge of the biological utilization of hydrocarbons as sources of energy and carbon is fragmentary. However, various investigators have proved that a biological oxidation of these compounds can occur. Since bacterial decomposition of hydrocarbons has been established, the fact is of significance in considering the carbon cycle. The percentage of carbon in hydrocarbons varies from 80 to 89 per cent; therefore, a significant amount of the world's carbon is combined in this form. The role of bacteria in the production of hydrocarbons is now widely accepted, although, according to Thayer (1931), there is no direct evidence that hydrocarbons of higher molecular weight than methane can be produced by their activity. 5 3. Industrial sources of hydrocarbons in waste water Hydrocarbon pollution in wastewater can come from a number of sources, such as petroleum, pesticides or other toxic organic matters that are discharged as effluents into water bodies (Abha and Singh, 2012). The presence of hydrocarbons in receiving water bodies is known to be carcinogenic, mutagenic and neurotoxic to living organisms, including plants and animals (Das and Chandran,2011). The major sources of hydrocarbon contaminants in wastewater effluents are oil spillage, automobile oils, pesticides, contaminated lands and urban storm water discharges. One of the main sources of hydrocarbon in wastewater is through oil spills. Oil spills are known to occur either accidentally through wrecks of tankers and equipment faults or deliberately through discharges, such as flushing tankers with sea water (Abha and Singh, 2012). Oil spills can also be caused by nature and human activities, when large amount of oil is spilled from oil seeps from ocean floors, as well as leaks that occur when petroleum products and other forms of oil are used on lands and later washed off into water bodies (Latimer et al., 1990). Another source of oil spill in water is leaking pipelines and underground oil storage tanks (Husaini et al., 2008). In addition, the emergence of various kinds of automobile or vehicles has brought about an increase in the use of automobile oil, which is a source of hydrocarbon pollution in wastewater. When automobile oil leaks or escapes from the car or drops on the ground, it could be washed through runoffs from rain into the water bodies, thereby causing pollution (USEPA, 1996; Husaini et al., 2008). With the onset of industrialization, the use and build-up of organic compounds have increased. Major sources which are responsible for organic contaminants are anthropogenic activities including the use of fuels, solvents, and pesticides. Various organic compounds are harmful and are related to health concerns globally. Diverse sources are responsible for the generation of hydrocarbons in sediments which are categorized below: • Anthropogenic sources • Petroleum inputs • Partial burning of fuels • Fires of forest and grass 6 • Biosynthesis of hydrocarbons by marine or terrestrial organisms • Diffusing from the petroleum source rocks, reservoirs, or mantle Organic pollutant is responsible for environmental and health-related problems; hence bioremediation provides an efficient explanation to this problem . Polycyclic aromatic hydrocarbons (PAHs) PAHs are considered to be ubiquitous contaminants. There are 100 diverse compounds of polycyclic aromatic hydrocarbons present. PAHs are seldom used for the industrial purpose, but only few are used for the manufacturing of pesticides, dyes, and plastics and for the production of medicines. Polycyclic aromatic hydrocarbons are produced on partial burning of organic matters. PAHs due to carcinogenic and mutagenic nature are highly poisonous to organisms. The degradation of PAHs is predominantly slow with high molecular weights because due to low hydrophobicity and water solubility it has a tendency to accumulate in sediments. PAHs have been classified as a priority pollutant by the USEPA which has classified 16 individual PAHs as pollutants due to its poisonous, carcinogenic, and mutagenic nature. Naphthalene Naphthalene is the simplest, most volatile and least toxic of the PAHs. In fact, naphthalene has been used in several research laboratories as a model to develop catalysts and biological process with potential to effectively destroy PAHs. Albeit naphthalene has a relative low solubility in water (32 mg/L) but it is highly hazardous. About 5% of all naphthalene disposed into the environment is released into water. Studies of naphthalene degradation may be significant because naphthalene is a common pollutant that serves as a chemical model for the degradation of PAHs. The range of naphthalene concentration in wastewaters is from ng/L to mg/L. Concentrations such as 1.65 mg/L were reported on wastewater samples from the radioisotope manufacturing facilities, 6–220 ng/L, in municipal wastewater, 285 mg/L (as naphthalene sulfonic acid), in effluents from ion-exchange resin towers, and 0.1–2.1 mg/L in dyeing and textile wastewater. PAHs like naphthalene and substituted naphthalenes are found to be the main components of petroleum-derived fuels, textile dyes, consumer products, pesticides (mothballs and insect repellents), plasticizers, and tanning agents, hence ubiquitously present in many ecosystems (Preuss et al., 2003). Recent reports have highlighted the accumulation of higher concentrations 7 of naphthalene in aquifer sediment, groundwater and subsurface soil, vadose zone, and river beds, signifying its bioaccumulation in the environment (Duttagupta et al., 2019, 2020). Polychlorinated biphenyls (PCBs) Polychlorinated biphenyls (PCBs) due to carcinogenicity, toxicity, and slow biodegradation in the nature are well thought-out to be the worst pollutants of commercial PCBs of about hundreds of thousands of metric tons are persevere in aquatic sediments. In adhesives and lubricants, dielectric fluids in flame retardants, transformers, hydraulic fluids, and plasticizers, PCBs are widely used. PCBs are released from disposal and spillage. Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) are still present in deep sediment layers which were deposited decades ago. Towards biotic and abiotic degradation processes, PCDD/Fs are often well-thought-out to be recalcitrant. PCDD/Fs are the most notorious pollutants present in nature. Another source of hydrocarbon pollution in receiving water bodies is pesticides. Pesticides include herbicides, fungicides and insecticides. When pesticides are applied to agricultural fields, only a little amount of them is said to reach their target while a significant proportion remain in the soil. During rainfall, the amount left in the soil is washed off into receiving water bodies (Ward et al., 1993). Of all pesticides, herbicides are indicated to be the most dangerous since they are applied directly on the soil to kill weed, leaving them more prone to be washed away by rain into water bodies. Also, contaminated land, which is lands that had former industrial activity or land where some kind of industrial activity has been carried out, is another source of hydrocarbon pollution in water. These lands could be contaminated by hydrocarbons and other organic chemicals, which can be washed away by rainfall into the water bodies thereby causing pollution. Furthermore, urban storm water discharges are indicated to be major sources of hydrocarbons in water. In urban communities, roads and car parks, which are often polluted with oil and gasoline from vehicles are large runoff producing. During rainfall, these pollutants are washed into drains and into receiving water bodies where they cause contamination. (Akpor et.al. 2014). 8 4. Effect of hydrocarbons on the environment Petroleum hydrocarbon contamination is one of the major environmental problems resulting from its large scale uses in transportation, industrial, agricultural and other sectors. Accidental releases and workshop seepage of petroleum products are of key concern for the environment. A variety of petroleum hydrocarbons such as crude oil, diesel, gasoline, heavy oil, kerosene etc. are used extensively as energy source, although their contaminations in soil and water have adverse effects. Contamination of soil with petroleum products deteriorates soil’s biochemical and physicochemical properties; it also limits the growth and development of plants. Oil spills has devastating effects on marine ecosystems, it hinders oxygen penetration in water which affects marine ecosystem. Petroleum hydrocarbon has several chronic and acute effects on human health as well. Inhalation, ingestion and dermal contact of these pollutants cause many harmful diseases (Ahmed and Fakhruddin, 2018). Hydrocarbon polluted wastewater has various impacts to the environment, which include reduction in crop yield, shortage of oxygen and effects on marine plants. When a farmland is irrigated hydrocarbon contaminated wastewater it leads to improper growth of crops, which could bring about reduction in crop yield and available food for households (Osuji and Nwoye, 2007; Ordinioha and Birisibe, 2013). The presence of oil in water can also reduce soil fertility to an extent that most of the essential nutrients are no longer available for crop utilization, which could lead to reduction in crop yield. A reduction in crop yield could also lead to a decrease in a farmer’s income (Emmanuel et al., 2006; Abii and Nwosu, 2009). Another environmental impact of hydrocarbon pollution is the shortage of oxygen. The majority of economic trees, which are main sources of oxygen in the environment, are known to depend on rainfall and sometimes water from water bodies for growth. In the presence of oil spill, the resulting oil which is denser than water reduces and in some cases prevents root penetration due to the hydrocarbons that fill the soil pores thereby expelling water and air. This in turn deprives the roots of the much-needed water and air (Henry and Heinke, 2005). This resultant effect of this is a distortion in the growth or death of such plants, thereby causing shortage of oxygen for human consumption (Edema et al., 2009). The presence of hydrocarbon in water prevents enough light from penetrating the water and gaseous exchange from taking place for utilization by marine plants. When this occurs, the plants are unable to photosynthesize, thus leading to their death and a resultant effect on the food 9 chain. In addition, plants may absorb hydrocarbon pollutants from water and pass them up to the food chain to consumer animals and humans (Gibson and Parales, 2000). 5. Removal necessity and purification methods Hydrocarbon contamination is of great worry because of their widespread effect on all forms of life. Pollution caused by increasing the use of crude oil is ordinary because of its extensive application and its related transport and dumping problems. Crude oil contains a complex mixture of aliphatic, aromatic, and heterocyclic compounds. Soil naturally consists of heavy metals, and due to human action like refining of oil and use of pesticides, their concentration in soil is rising. Several areas have such high heavy metal and metalloid concentration that surrounding natural ecosystem has been badly affected. The reason is that heavy metals and metalloids limit microbe’s activity rendering it unsuitable for hydrocarbon degradation, thus reducing its effectiveness. Environmental remediation is thus extremely necessary and involves with the elimination of pollutants from soil, air, and water. In the last several decades, different methods have been employed and applied for the clean-up of our environment which includes mechanical, chemical, and biochemical remediation methods (Srivastava et al, 2019). Potential oil sources are petroleum refineries, oil production wells, oil and gas fields, petrochemical industries, gasoline dispensing stations, transport vehicles carrying petroleum products, and/or leaking oil/gas pipelines, which contaminate/pollute water bodies and soil through spills or wastewater discharges. The petroleum, metallurgical, and transportation industries are concerned about the separation and recovery of oils from water and the treatment of oily wastewater. Petroleum refineries and oil/gas fields usually have a high concentration of oil in miscible and immiscible forms in the effluent. During the production of oil from oil wells/reservoirs, an increased amount of coproduction either oil-in-water (o/w) or water-in-oil (w/o) emulsions happens. Transportation of water along with oil increases additional energy expenditure and restricts the volumetric flow of oil. Several water desalinations plants or reverse osmosis (RO) treatment units face serious problems due to the presence of oil in their influent water sources. The effluent wastewater must meet the discharge standards set by the environmental regulating agencies like Central and/or State Pollution Control Boards and other regulatory bodies. The Ministry of Environment and Government Forest (MOEF), India, has set the discharge limit of oil to wastewater as less than 5 mg/l (MOEF Notification 2008). The formation of emulsions (o/w or w/o) significantly alters the characteristics of effluent wastewater. 10 With respect to effects on human, the highly toxic hydrocarbons such as the polycyclic ones are indicated to pose serious threats to the human body. When ingested, they damage organs and systems, such as the respiratory system, nervous system, immune system, circulatory system, reproductive system, kidney, liver etc (Ordinioha and Birisibe, 2013). The extent of damage is dependent on the level of exposure and the susceptibility of the individual (Abha and Singh, 2012). Other impacts of hydrocarbon polluted effluents to humans include the risk of cancer, hormonal problems that can disrupt reproduction and developmental processes (Urum et al., 2004; Mbhele, 2007; Aguilera et al., 2010). Methods for removal The latest methods of removing and eliminating oil pollution from the sea include aeration; adsorption processes with the help of activated carbon and natural and synthetic adsorbents; oxidation by some oxidants; the use of laser technology; the use of dispersants; adsorption and separation of water surface by various types of adsorbents, especially adsorbents prepared using nanotechnology; evaporation of petroleum compounds using vacuum techniques; physical separation by means of foaming; conversion of oil pollution to other compounds by methods such as photo-oxidation as well as biological methods. A high set-up and exploitation cost, the need for skilled personnel to set up and operate, the lack of rapid adaptation of microorganisms to remove these compounds, the presence of electron receptors, and the production of secondary toxic compounds have limited the use of these methods. A few of the removal techniques and methods are listed below. Canvas The use of canvas is now commonly used to capture and seal leakage of oil. These canvases are designed to prevent floating oil from escaping the surface of the water. One type of canvas, called a canopy fence, consists of a PVC barrier that is fitted underneath the polyethylene floats and is provided with apertures for holding the barrier at the bottom. In some of its types, 6foot-long hinges are used, with a total length of 50–100-feet in length. Chemicals One of the methods for concentrating oil is the use of chemicals at contaminated water levels that reduces the rate of oil release and causes oil returns at the level of the oil mass. This method is used in real-world oil spills, but only in calm water. 11 Adsorption Method Sorbents, materials, or mixtures of insoluble materials are used to recover fluids during adsorption, physical attachment, or both. Adsorbents must be absorbent of oil compounds and also waterproof. Although they may be used as the only cleaning method for small leaks, adsorbents are often used to remove the final oil trap. Also, the benefits of using adsorbents are to limit the speed of the playback of the layer and help clean it. Debris can be divided into three basic groups: organic, natural, and combined organic. Natural organic adsorbents include carbon monoxide, nitrogen, dry grass, sawdust, corn wood, feathers, and other products, mainly carbon-readable. Organic adsorbents can absorb between 3 and 15 times the weight of their oil, but their use also has some disadvantages. Some organic adsorbents tend to absorb water as much as absorbing oil. This causes the absorbent to drown. Many organic adsorbents lose particles such as sawdust, which is difficult to collect after being spread over the surface of the water. Natural mineral adsorbents can absorb oil from 4 to 20 times their weight. Mineral absorbers, like organic adsorbents, are inexpensive and readily available in large amounts. These types of adsorbents are not used on the surface of the water. Combined absorbents include human-made materials that resemble plastics, such as polyurethane, polyethylene, and polypropylene, which are designed to absorb fluids on their surface (such as sponges). Other compound absorbents include shrink-bonded polymers and rubber materials, which absorb fluids on their solid structure, causing the swelling of absorbent materials. Most composite adsorbents can absorb oil up to 70 times their weight. The properties of both absorbent and oil types should be considered when selecting adsorbents to clean up leakages. Oil adsorption with light petroleum products is faster. Also, the oils with thicker layers are more effective at attaching surfaces. Lightweight oil with less viscosity than the heavier and more viscosity type is easier to handle during retrieval of sealing materials, which results in secondary contamination . Johnson and co-workers suggested the potential of using linen fiber to clean up oil spills (Johnson et al.,1973). Advanced and Chemical Oxidation The conventional oxidation treatment involves the addition of an oxidizing agent to water or a solution containing contaminants. After such simple chemical oxidation, the pollutants are decomposed and removed. Usually, chemical oxidation in wastewater treatment means adding oxidizing agents such as ozone, hydrogen peroxide, permanganate, chlorine dioxide, chlorine 12 (Cl2, HOCl), and oxygen to the sewage to change the chemical composition of one or more pollutants. Also, some of the most important applications of oxidation in sewage management have been to reduce the amount of bacteria and sewage viruses in order to control the odour and eliminate ammonia and solutes. Chemical oxidation is also effective for the removal of odorous compounds such as sulphides and mercaptans. In addition to the aforementioned uses of chemical oxidation, in order to improve the biological purification ability of organic or nonbiodegradable organic compounds, the removal of inhibitory effects of some organic and inorganic materials on microbial growth, reducing or eliminating the toxicity of certain organic and inorganic materials on microbial growth and aquatic plants are used to remove BOD, COD, ammonium, and resistant organic compounds. Types of chemical oxidation treatment methods are purification by conventional oxidation and purification by advanced oxidation methods. Advanced oxidation processes (ozone, ozone, hydrogen peroxide, ozone/ultraviolet, hydrogen peroxide/ultraviolet) are suitable alternatives for decomposing organic biodegradable organic waste from conventional wastewater treatment. The advanced oxidation process has significant advantages (such as the lack of high levels of sludge production) compared to conventional filtration methods. This method usually produces hydroxyl radicals at ambient temperature, which unintentionally attacks all organic and inorganic pollutants in the sewage system. Since advanced oxidation processes are expensive and have high operating costs, in recent years, a new approach to these processes, called catalytic ozonation processes (COP), has been considered. Recently, various metals such as iron, aluminium, zinc, chromium, and copper have been used as catalysts to enhance the efficiency of advanced oxidation processes. One of the benefits of advanced oxidation is that the processes are generally able to completely destroy specific substances. Therefore, using a biological method as a final treatment will complete the process. Heterogeneous photocatalysts, along with semiconducting processes, extinction, Fenton and sonolysis and similar processes are more efficient than other processes such as wet oxidation and electro dialysis. However, most studies have been conducted with the model of aqueous solutions and surface water (including rivers and lakes), while less attention has been paid to the actual wastewater from wastewater treatment plants or effluent from industrial units. Fenton (or the photo-Fenton oxidation process) or Fenton’s reaction is a non-expensive and environmentally friendly oxidation method, which was discovered by Fenton in 1894 when he 13 strongly improved tartaric acid oxidation with the use of ferrous ion (Fe2+) and H2O2 . This process is widely used in wastewater treatment. Fenton’s reagent is a solution of H2O2 and ferrous ions with a complex mechanism, which can be simplified by the following reactions : Briefly, the reaction between ferrous ions and H2O2 produces hydroxyl radicals with high oxidative power (a) that attack the organic compounds present in the water (b). Unfortunately, some parallel reactions occur (d–f), and so the hydroxyl radicals are not only consumed to degrade the organic matter but also to produce other radicals, with less oxidative power, or other species (scavenging effect of HO•). In addition, this leads to the undesired consumption of H2O2 (d). On the other hand, reaction (g) and (h) indicate generation of Fe2+ by the reaction between H2O2 and Fe3+ (Fenton-like process); this way, ferrous ion is restored, acting as a catalyst in the overall process. This briefly summarises the working mechanism for Fenton’s reaction. 6. Bioremediation Bioremediation is known as the use of natural microorganisms in the correction of a contaminated environment (Sharma, 2012). The process uses naturally occurring microorganisms such as fungi, yeast and bacteria for the breakdown or degradation of hazardous substance into less toxic or non-toxic substances (Mbhele, 2007). It is also a process whereby microorganisms degrade and metabolize chemical substances and restore environment quality (Dave and Ghaly, 2011). Oil spill causes contamination of soil which is considered as the chief worldwide concern. Pollution of soil due to petroleum causes a serious effect to human being, affects the groundwater, decreases the agricultural production of the soil, and causes economic loss and ecological problems. Plants, animals, microorganisms, and humans are affected by the toxicity of the petroleum hydrocarbons. Oil spill and accidents 14 occur due to the transportation of crude oil which is generally through tankers on water or through land pipeline. Problems of the oil contamination occur mostly due to the reason that the main oil-producing countries are not the chief oil clients; hence petroleum is transported to the consumption area. Certain microorganisms are accountable for the petroleum hydrocarbon degradation and are used as the resource of carbon and energy for growth and maintenance. Soil contamination can be remediated by many ways including both physicochemical and biological techniques. Bioremediation process involves the utilization of natural microorganisms for the decontamination of atmosphere, this process converts pollutants into useful or nontoxic substances by using bacteria, fungi, and yeast which are the naturally occurring microorganisms. This is also a process in which microorganisms restore the quality of the environment by degrading and metabolizing the chemical substances, Table 1 represents the main microorganisms which are included in the remediation of hydrocarbons. Bacteria Yeast and fungi Achromobacter Acinetobacter Alcaligenes Aspergillus Candida Cladosporium Arthrobacter Bacillus Brevibacterium Corynebacterium Flavobacterium Nocardia Pseudomonas Vibrio Penicillium Rhodotorula Sporobolomyces Trichoderma viride Fusarium Trichoderma harzianum Table 1. (List of microorganisms for bioremediation) Source: Zhu et al. (2001) Role of microorganisms for the removal of hydrocarbons: Microbes have a fundamental role in the remediation of polluted water by Floating treatment wetlands (FTWs).The bacteria are attached to the roots form biofilms through a repeated proliferation process. The oxygen and exudates released by the plants create a substrate for microbial growth and colonization on the root beneath the water level. Thus, along the vegetation, the performance of FTWs also depends upon the metabolism of the microbial community in water, attached to the roots and floating mats. The application of plants in 15 combination with microorganisms in FTWs is an effective and sustainable approach for the treatment of wastewater. The plant–microbe interaction enhances the efficacy of FTWs. Although the plant–bacteria interaction plays an essential role in the removal of contaminants from aquatic ecosystem, the interaction of the plant with bacteria in the FTWs is not well explored.( Shahid et.al , 2020). Bacteria have a unique ability to form biofilms, also known as epiphytic microbes. Biofilm formation begins with the attachment of free-floating microbes to gas–liquid and solid–liquid interfaces. These biofilms have a key role in the assimilation of the biogeochemical cycles and the dynamics of an ecosystem process. In the aquatic ecosystem, aquatic plants are an essential substrate for the establishment, growth, and development of biofilms. Aquatic plants release oxygen, essential for aerobic bacteria attached to roots, and stimulate the nitrogen cycle in the roots’ surroundings. Biofilms are composed of an extracellular matrix comprised of polysaccharide biopolymers, proteins, and DNA that hold the cell together. The structural integrity of biofilms is obtained by secreted proteins, various types of exopolysaccharides and cell surface adhesions. The development and maintenance of these biofilms rely on small molecules such as homoserine lactones, antibiotics, and secondary metabolites, such as the Staphylococcus aureus matrix, provide proteins for the synthesis of biofilm. The extracellular matrix also facilitates the formation of adhesive protein found anchored to the cell wall of S. aureus, holding the cells together within the biofilm by interaction with other proteins. The extracellular DNA also strengthens the structural integrity of the biofilms. For example, Pseudomonas aeruginosa contains a significant amount of DNA to provide stability to biofilms. The nature of biofilms and associated matrices depends upon the types of substrates, medium, and growth conditions. Bacillus subtilis, a Gram-positive bacterium, can make biofilms via production of two different polymers: polysaccharide extracellular polymeric substances and poly-d-glutamate. Both of these polymers contribute to biofilm formation; however, the contribution of each polymer is determined by strain and prevailing conditions. The plants can also modify the function and structure of the microbial community in their rhizosphere . The biodiversity and species of bacteria determine the functions of the biofilms. The biofilm-forming bacteria have been reported as diverse and host specific. The secretion of macrophytes and growth status can determine the bacterial composition of biofilms in the aquatic ecosystem. Moreover, the bacterial community of biofilms was found to be different than those in the surrounding water column. 16 Microbes are known as bio-remediators due to their capability to break down virtually all classes of organic pollutants. Microbes degrade the organic pollutants by a process of cometabolism. In this process, microbes in the rhizospheric zone of aquatic and terrestrial plants degrade the complex carbon-based compounds in order to obtain organic carbon and electron acceptors. In natural water, the biodegradation rate depends upon the microbial population and amount of xenobiotics, and the numbers of the microbes are heavily influenced by the macrophyte species. Plants give organic carbon to microbes present in the rhizosphere that assist them to degrade complex organic compounds, such as hydrocarbons and aromatic hydrocarbons. Bacteria also release indole acetic acid (IAA) to improve plant growth. Many bacteria isolated from aquatic plants also showed pollutant degradation and plant growthpromoting activities. The biofilms attached to aquatic plants are capable of degrading organics such as phenolics, amines, and aliphatic aldehydes. Additionally, these biofilms are capable of degrading dissolved organic matter such as polychlorinated biphenyls (PCBs) and atrazine. The aquatic plant rhizosphere is also enriched with methanotrophs containing a collection of Proteobacteria, which utilize methane for obtaining carbon and energy. Methanotrophs can degrade numerous types of harmful organic complexes such as chlorinated ethenes by enzymatic reactions. The Eichhornia crassipes can remediate eutrophic water by influencing the production of gaseous nitrogen. Aerobic and anaerobic degradation of wastewater hydrocarbons Hydrocarbon-degrading microorganisms being ubiquitous in the world's oceans (Head et al., 2006; Yakimov et al., 2007), biodegradation mediated by indigenous microbial communities is the ultimate fate of the majority of oil hydrocarbon that enters the marine environment (Leahy and Colwell, 1990; Prince, 2010; Atlas and Hazen, 2011). In response to the natural complexity of hydrocarbon compounds found in petroleum deposits, diverse marine microorganisms have evolved with an equal complexity of metabolic pathways to take advantage of hydrocarbons as a rich carbon and energy source. To minimize the environmental impact of oil spills and to optimize the environmental benefits of biodegradation, it is essential to uncover the metabolic potential of hydrocarbon-degrading bacteria and to address the factors that limit microbially-catalysed biodegradation in situ. 17 Pathways for degradation of aromatic hydrocarbons The hydrophobicity and chemical stability of aromatic hydrocarbons, described above, give negligible biological activity to these molecules. Therefore, to break them down, in either aerobic or anaerobic conditions, bacteria need to destabilize the benzene ring through reversible and irreversible chemical modifications (Díaz et al. 2013). In both aerobic and anaerobic pathways of aromatic hydrocarbon biodegradation a Terminal Electron Acceptor (TEA) is required. TEA determines the energy balance and the metabolic reaction used by microorganisms (Philipp and Schink ,2012 ; Schink et al.2000). However, studies with microcosms and stable isotope probing (SIP) have shown that, in environments dominated by a particular TEA, the dominant bacterial strain was not specialized to degrade the aromatic hydrocarbon being evaluated (Kleinsteuber et al. 2012 ; Pilloni et al. 2011 ). These results suggest that aromatic-degrading strains are specialized and the dominant bacterial strains are generalists and are able to use compounds other than aromatic hydrocarbons as carbon and energy sources (Staats et al. 2011) Under aerobic conditions In aerobic conditions, the first step of upper pathways is an oxidation catalysed by monooxygenases (hydroxylases) or by dioxygenases (Huijbers et al.2014 ; Parales and Resnick 2004 ). The monooxygenases catalyse the cleavage of the oxygen-oxygen bond of O2 inserting one oxygen atom into the aromatic ring while the other is reduced to H2O.These enzymes are classified in eight groups according to their structure, sequence, type of reaction, type of reaction catalysed and type of electron donor. Monooxygenases can oxidize both monoaromatic and polyaromatic hydrocarbons. Some examples are phenol hydroxylase and toluene/o-Xylene monooxygenase catalysing benzene and phenol oxidation as reported by Cafaro et al. (2004) from Pseudomonas stutzeri OX1 and 4-hydroxyphenyl acetate 1-monooxygenase catalysing oxidation of 4-hydroxyphenyl acetate as reported by Hareland et al. (1975) from P.acidovorans . In the case of PAHs, there was reported oxidation of naphthalene and fluorene catalysed by a toluene 4monooxygenase following a similar route to monoaromatic hydrocarbons. (Fig 3) 18 Fig 3: Processes of oxidation, a- benzene, b-4-hydroxyphenyl acetic acid, c-naphthalene d-fluorene (Cafaro et. al,2004) Under anaerobic conditions In the absence of oxygen, oxidized inorganic compounds such as nitrate (NO 3=1), manganese (Mn(IV)),iron (Fe(III)), sulphate (SO4-2) and carbon dioxide (CO2) act as the terminal electron acceptors (TEAs) (Fuchs et al. 2011 ; Vogt et al.2011). The methanogenic reduction also plays an important role in anaerobic biodegradation, particularly at sites that have been contaminated for longer periods of time where other TEAs have been depleted. Currently, the following five ways of activation of aromatic hydrocarbons activation are being discussed: (1) Phosphorylation: Activation occurs by insertion of a phosphate group. In the case of phenol in Tauera aromatica catalysis occurs by a phenyl-phosphate synthase (Narmandakh et al. 2006 ;Schmeling et al.2004 ). (2) Fumarate insertion: Alkylated aromatic hydrocarbons such as toluene, cresols and xylenes are activated by radical-based addition of a fumarate to the methyl group and, in the case of ethylbenzene onto the side chain (Heider, 2007). (3) O2-independent hydroxylation: Vogel and Grbìc-Galìc (1986) using partially 18 O-labelled water in an enrichment culture in methanogenic incubations presented toluene and benzene hydroxylation indicating that the hydroxyl group originated from water. Johnson et al. (2001) showed that an ethylbenzene dehydrogenase in Azoarcus sp. strain EB1 catalyses the insertion of one hydroxyl group onto ethylbenzene to produce (S)-(−)1-phenylethanol (Fig.3) (4) Carboxylation: Also called the “biological Kolbe-Schmitt reaction”, the carboxylation reaction has been suggested for monoaromatic hydrocarbons and non-substituted PAHs as follows. The carboxylation of phenol in para-position yielding 19 4-hydroxybenzoate by strains K172 and S100 was proposed by Tschech and Fuchs (1987). Zhang and Young (1997) working with enrichment cultures with 13C bicarbonate and sulphate reducing conditions found 2-naphthoate and phenanthrene carboxylic acid indicating that naphthalene and phenanthrene carboxylation. (5) Methylation: Also called Friedel-Craftstype methylation, it was reported by Safinowski and Meckenstock (2006) as the initial reaction in the anaerobic degradation of naphthalene by sulphate reducing enrichment cultures. Fig 4: Current 5 activation ways studied for anaerobic activation (Heider,2007) Factors affecting bioremediation It has been reported that many factors are affecting the rate of bioremediation which include energy sources, bioavailability as well as bioactivity and biochemistry of the systems. The major factors affecting bioremediation are presented in Table 2. Microbial Growth until critical biomass is reached Mutation and horizontal gene transfer Enzyme induction Enrichment of the capable microbial populations Production of toxic metabolites Environmental Depletion of preferential substrates Lack of nutrients Inhibitory environmental conditions Substrate Too low concentration of contaminants 20 Chemical structure of contaminants Toxicity of contaminants Solubility of contaminants Biological aerobic versus anaerobic process Oxidation/reduction potential Availability of electron acceptors Microbial population present in the site Growth substrate versus co-metabolism Type of contaminants Concentration Alternate carbon source present Microbial interaction (competition, succession, and predation) Physico-chemical bioavailability of pollutants Equilibrium sorption Irreversible sorption Incorporation into humic matters Mass transfer limitations Oxygen diffusion and solubility Diffusion of nutrients Solubility/miscibility in/with water Table 2: : Major Factor Affecting Bioremediation (Luka Y.et.al,2018) Microorganisms can be isolated from almost any environmental conditions. Microbes will adapt and grow at sub-zero temperatures, as well as extreme heat, desert conditions, in water, with an excess of oxygen, and in anaerobic conditions, with the presence of hazardous compounds or on any waste stream. The main requirements are an energy source and a carbon source. Generally, degradation process relies on microbial (biomass concentration, population diversity, enzyme activities), substrate (physico-chemical characteristics, molecular structure, and concentration), and a range of environmental factors (pH, temperature, moisture content, availability of electron acceptors and carbon and energy sources) as depicted in Table 2. These parameters affect the acclimation period of the microbes to the substrate. The molecular structure and contaminant concentration have been shown to strongly affect the feasibility of bioremediation and the type of microbial transformation occurring, and whether the compound will serve as a primary, secondary or co-metabolic substrate. The bioavailability of a contaminant is controlled by a number of physico-chemical processes such as sorption and desorption, diffusion, and dissolution. A reduced bioavailability of contaminants in soil is caused by the slow mass transfer to the degrading microbes. Contaminants become unavailable when the rate of mass transfer is zero (0). The decrease of 21 the bioavailability in the course of time is often referred to as aging or weathering . It was further explained that the ability of organisms to transfer contaminants to both simpler and more complex molecules is very diverse. In light of our current limited ability to measure and control biochemical pathways in complex environments, favourable or unfavourable biochemical conversions are evaluated in terms of whether individual or groups of parent compounds are removed, whether increased toxicity is a result of the bioremediation process, and sometimes whether the elements in the parent compound are converted to measurable metabolites. These biochemical activities can be controlled in an in-situ operation when one can control and optimize the conditions to achieve a desirable result. 7. Bioremediation over conventional processes Among the various techniques used for clean-up actions, bioremediation seems to be the most acceptable and economically justified. Composite mixture of diverse chemical substances makes up the crude oil. Oil and its component are recognized by microbes using bioemulsifiers and biosurfactants, and then they join themselves; hydrocarbon is used as the resource of carbon and energy. High molecular weight hydrocarbons due to their low adsorption and solubility limit their accessibility to microorganisms. Oil biodegradation rates are improved by the biosurfactant’s addition which increases the elimination and solubility of these pollutants. The oil constituents vary particularly in susceptibility, volatility, and volubility to biodegradation. A number of substances are easily degraded, some are non-biodegradable, and some oppose degradation. Diverse species of microbes preferentially attack diverse compounds due to this biodegradation of petroleum that occurs at different rates but concurrently. Enzymes produced by microorganisms in the presence of sources of carbon are accountable for attacking the hydrocarbon molecules. Hydrocarbon present in the petroleum is degraded by different enzymes and metabolic pathways. Hydrocarbon degradation is prevented by the lack of suitable enzyme. Biological techniques are more economical and proficient than physicochemical techniques. The degradation rate of petroleum products is increased by developing several remediation methods. Bioremediation through microorganism is considered to be the most effective method in comparison to other biological methods, but the high molecular weight hydrocarbons with 22 low adsorption and solubility limit their accessibility to microorganisms. A major advantage of bioremediation over other remediation processes is the low cost involved and the savings in the time put forth by workers to clean a contaminated site. Also, bioremediation allows for savings in that it continues to clean the contaminated site without the constant need of workers. This saves a great deal of money which would be spent on labour hours (Zhu et al., 2001). The process is also indicated to be environmentally friendly. This is because no foreign or toxic chemicals are added to the site, hence does not allow for any disruptions to the natural habitat (Dave and Ghaly, 2011). It allows for the natural organisms to degrade the toxic hydrocarbons into simple compounds which pose no threat to the environment and this also eliminates the need to remove and transport the toxic compounds to another site. Despite the advantages, some of the drawbacks of bioremediation remediation are that it is slow and depending on where the pollution occurs, it may be difficult to provide the proper nutrient concentration to the oil degrading microorganisms (Swannell et al., 1996; Dave and Ghaly, 2011). Analysis of recent reports regarding unsuccessful bioremediation attempts indicates that there is a need to highlight the fundamental aspects of hydrocarbon microbiology in a clear and concise manner. Therefore, it is important to elucidate some crucial, but often overlooked, factors such as, the formation of crude oil and abundance of naturally occurring hydrocarbons, bacterial ability to not only survive but also to utilize such compounds as an attractive energy source, the significance of nutrient limitation on biomass growth in context of bioremediation efficiency and the formation of aerobic and anaerobic conditions, as well as the role of surfactants for maintaining appropriate C: N:P ratio during initial stages of biodegradation etc. (Ławniczak et.al. 2020). Bioremediation for removal of Naphthalene Various physical, chemical, biological, and their combined technologies have been attempted to remediate organic-contaminated waters. The in situ microbial degradation of Polycyclic aromatic hydrocarbons (PAHs) like naphthalene is limited by their low bioavailability and low water-solubility. Among various attempts made to treat such wastewater, such as, evaporation, polymeric absorption, solvent extraction, and conventional biological treatment, are proved ineffective due to the quite stable structure of the naphthalene ring and the high concentrations of salt and acid in the streams from the dye manufacturing process. The methods for treating PAHs mainly include biodegradation, scrubber absorption, high-energy electron beam irradiation, ozonation, catalytic combustion volatilization, photo-oxidation, chemical oxidation 23 and adsorption. Most of the PAHs such as naphthalene are not aerobically degraded in activated sludge system, as the benzene rings is susceptible to reduction under low redox potential. The oxic/anoxic (O/A) system is an alternative to the traditional activated sludge process for treating high naphthalene solution. Recently, a number of oxic/anoxic bacteria have been used to biodegrade naphthalene with several pathways and metabolic diversities described. Bacteria such as Pseudomonas putida, Rhodococcusopacus, Mycobacterium sp., Nocardia otitidiscaviarum, and Bacillus pumilus have been reported to biodegrade naphthalene. Pseudomonas aeruginosa is an environmental bacterium that can be isolated from many different habitats, including water, soil, and plants, but it is also an opportunistic human pathogen causing serious nosocomial infections. Pseudomonads are the best-known bacteria capable of utilizing hydrocarbons as carbon and energy sources and producing bio surfactants which enhance the uptake of such immiscible hydrophobic compounds. 8. Permissible limits of hydrocarbons in water WHO produces international norms on water quality and human health in the form of guidelines that are used as the basis for regulation and standard setting, in developing and developed countries worldwide? The guidelines developed by WHO are prepared through a vast global consultative process involving WHO member states (India is the member state), national authorities and international agencies, in consultation with the WHO Expert Advisory Panel. Hydrocarbons Standard limits as per WHO guidelines (mg/L) Acrylamide 0.0005 Alachor Aldicarb 0.02 0.01 Aldrin and Dieldrin 0.00003 Atrazine 0.002 Benzene 0.01 Benzopyrene 0.0007 Bromodichloromethane (BDCM) 0.06 Bromoform 0.1 Carbofuran 0.007 24 Chloroform 0.3 Chlorotoluron 0.03 Chlorpyrifos 0.03 Cyanazine 0.0006 Cyanide 0.07 1,2-Dichlorobenzene 1.0 1,4-Dichlorobenzene 0.3 1,2-Dichloroethane 0.03 Dichloromethane 0.02 2,4-Dichlorophenoxyacetic acid 0.03 1,2-Dichloroethylene 0.05 1,2-Dichloropropane 0.04 Dimethonate 0.006 1,4-Dioxane 0.05 Monochloroacetate 0.02 N-Nitrosodimethylamine 0.0001 Pentachlorophenol 0.009 Pyriproxyfen 0.3 Styrene 0.02 Terbuthylazine 0.007 Tetrachloroethylene 0.04 Toluene 0.7 Trichloroacetate 0.2 Trichloroethylene 0.02 2,4,6-Trichlorophenol 0.2 Trifluralin 0.02 Table 3 (Source: CPCB, WHO Specifications, 2019) 25 9. Literature review Various methods have been implemented for the removal of hydrocarbons from wastewater, of which bioremediation is the most suitable, as discussed earlier (both from an economic and environmental point of view). A wide variety of bacteria and fungi like Achromobactor sp (Hong et al.2017), Trichoderma (Dacco et al. 2020), Flavobecterium (Chaudhary et al. 2019), Penicillium (Vanishree et al. 2014), Bacillus (Banerjee and Ghoshal, 2017) etc. have been studied for the purpose of removal of both polycyclic and acyclic hydrocarbons present in wastewater, with the help of bioremediation processes. Dacco et al. in the University of Pavia, Italy in 2020, worked with the fungi Trichoderma and explored its possibilities as a bioremediation agent and their ability to grow on used engine oil. The morphological analysis of the selected strains based on observations under a light microscope of the conidia and conidiophore morphology led to the identification of the four strains as F1020 T. asperellum and F12, F26, and F58 T. harzianum. Moreover, the sequencing of the ITS regions, compared with target sequences with BLAST, confirmed this morphological identification. The tree obtained in the phylogenetic analysis contained 32 taxa. The tolerance test showed that all strains could grow on used engine oil, meaning that the pollutant does not totally inhibit their growth and that they could use it as a nutrient source. However, there were differences in the growth of the strains. T.Harzianum F26 was the best performing, while T. asperellum F1020 was the worst. The experiments carried out in this work showed that the Trichoderma strains tested, could tolerate the presence of used engine oil and that they can use it for their growth. The degradation efficiency, however, varies greatly depending on the single strains and species, as demonstrated in GC/MS tests. The causes may be different environmental growing conditions and substrate types, but especially adaptation to the presence of toxic agents such as pesticides, PAHs, and chlorinated agents. In 2017, Banerjee and Ghoshal. (Indian Institute of Technology, Guwahati) worked with pure cultures of Bacillus cereus for bioremediation of petroleum wastewater. They treated the wastewater samples collected from oil refineries and oil exploration sites by hyper phenoltolerant Bacillus cereus (AKG1 and AKG2) in laboratory-scale batch process to assess their bioremediation efficacy. Quality of the treated wastewater samples were analysed in terms of removal of chemical oxygen demand (COD), total organic carbon (TOC) and ammonium nitrogen content, and improvement of biological oxygen demand (BOD). The bacterial 26 strains were used in 5 different ways –free cells of AKG1, free cells of AKG2, free cells of co-cultured AKG1 and AKG2, immobilized AKG1 and immobilized AKG2 – for the biodegradation of wastewater samples over a period of 20 days. During microbial treatment of petroleum wastewater samples, in addition to COD removal, prominent increase in BOD was also observed. After 20 days of bioremediation, initial BOD values of 180 mg/L (sample I) and 420 mg/L (sample II) increased to as high as 861 mg/L and 1532 mg/L, respectively. The ratio of BOD to COD (an index of biodegradability performance) of wastewater samples was very low before bioremediation (0.030∼0.065) which indicated difficulty of treating them by biological methods. However, after treatment, the ratio was increased to 0.89 and 0.79 for sample I and sample II, indicating significant improvement of biodegradability of the samples. The reduction in total organic carbon (TOC) present in wastewater samples also followed similar trend as that of COD. Without any external N2 source, removal of TOC was found to be in the range of 30%- 83%. The TOC level was reduced by 45–84% and 35–77% in presence of organic and inorganic N2 sources, respectively. Among the different combinations of bacterial cultures used the co-culture of AKG1 and AKG2 showed the best performance in degrading the wastewater samples. In 2015, Karimi et.al at the Arak University of Medical Sciences, Arak, Iran worked with Pseudomonas aeruginosa to evaluate the applicability of an anoxic/aerobic system for the biological treatment of naphthalene. They also investigated the growth conditions of P. aeruginosa in anoxic-aerobic reactor, that constitute the most important factor affecting biodegrading efficiency, kinetics, and the biodegradation mechanism of naphthalene. Pseudomonas aeruginosa was counted on R2A medium and MSM containing 30 mg/L naphthalene, according to Standard Plate Count Method. Pseudomonas aeruginosa bacteria PTCC 1707 growing on the above medium (with naphthalene and sugar as the only carbon source) were defined as naphthalene degrading bacteria. Then bacterial suspension with a specific turbidity (0.5-10 NTU) was prepared from growing bacteria. Various factors were examined for their influence on growth of selected strain and on the biodegradation of naphthalene. These included temperature, pH, nitrogen concentration, salinity, inoculum concentration, different naphthalene concentration and SCOD. The cultures were incubated in a shaking incubator at 150 rpm, 30°C. The naphthalene concentration after degraded in various samples was determined using gas chromatography. All experiments were carried out in triplicate. The degree of naphthalene degradation was calculated as: 27 Degradation efficiency (%)= 𝐶0 −𝐶𝑡 𝐶0 ×100 Where C0 and Ct stand for the naphthalene concentrations before and after degradation, respectively. To examine the growth of P. aeruginosa (OD and CFU/mL) in vertical anoxic–aerobic continuous flow combined bioreactor and tolerance of naphthalene by P. aeruginosa, the cells were cultivated in nutrient broth with concentration of naphthalene. It appeared that the cells were able to survive naphthalene concentration as high as 20 mg/L naphthalene. Figure 5 shows the growth curves of P. aeruginosa AT18 in the presence of naphthalene, this can be attributed to growth of biomass on naphthalene intermediates. Fig 5: Growth of P. aeruginosa (OD and CFU/ mL) in vertical anoxic– aerobic continuous flow combined bioreactor with 0.1, 0.5, 1, 5, 10 and 20 mg/ L for naphthalene. Pseudomonas being a gram-negative rod-shaped bacterium with high ability in decomposition of organic and oil pollutants, including naphthalene, its ability depends on production of catalytic enzymes and formation of metabolic paths. Due to presence of various degrading enzymes in bacteria, they have stronger effect on naphthalene degradation process relative to other microorganisms. In this particular study, following culture of bacterium Pseudomonas aeruginosa and formation of the microbial suspension from it and having accomplished the compatibility process between this microorganism and naphthalene, they were blended in a combined anoxic/aerobic reactor. As the solid retention time increased from 2 days to 8 days, COD and naphthalene removal efficiency gradually increased. On day 8, removal efficiency of naphthalene and COD reached 94 and 96.1%, respectively. This work highlighted the link between the Pseudomonas aeruginosa and the degradation of 28 naphthalene as PAH in a wastewater subjected to anoxic/oxic condition on vertical anoxic– aerobic continuous flow combined bioreactor. It was seen that the degradation of naphthalene rises to 94% within 8 days under optimum conditions (temperature 27°C, pH 8.0, and naphthalene concentration of 20 mg/L). Naphthalene concentration, retention times, flow rate, pH and turbidity formed by bacterium in the bioreactor were shown to be of particular importance in the removal of naphthalene. Naphthalene degradation increased with the increasing naphthalene concentration to 20 mg/L. Alkaline pH 7,8 was favourable for Pseudomonas aeruginosa for naphthalene degradation. More than 96% of naphthalene and 76% of COD was degraded when the pH of the bioreactor ranged from 7 to 8. Increase in dose of bacterium injection into the system is goes along with increase of naphthalene degradation. At pH 8 and 10 NTU which is the greatest amount of bacterium injected to the system, the highest naphthalene removal efficiency was obtained. As the initial concentration of naphthalene increased from 0.5 to 20 mg/L, the remaining concentration of naphthalene decreased from 65.5% to 33.4% after 3 days. Based on experimental results, it was determined that this process can effectively reduce naphthalene under optimal conditions and this method can be used for the removal of similar compounds. In 2017, Abo-State et.al at Cairo University, Egypt worked with Bordetella avium strains to study the bioremediation of naphthalene isolated from petroleum refinery waste water. Wastewater and Sludge samples polluted with petroleum oil from Cairo Oil Refining Company (CORC), were used for isolation of indigenous bacterial communities. The isolation of bacterial strains followed four steps of adaptation and enrichment technique for selection of the most naphthalene tolerant bacterial strains. Screening on four Naphthalene concentrations to determine the most potent strains having the abilities to use Naphthalene as a sole carbon and energy source were conducted. The most potent bacterial isolates were MAM-P9, MAM-P14, MAM-P22, MAM-P25 and MAM-P26. The abilities of the five most potent bacterial isolates to grow on BSM amended with 4,5,6 and 7 mM Naphthalene were determined by recording their growth (O.D) and secretion of extracellular protein after zero time (initial), 1,2,3,4,5,6,7,14 and 21 days spectrophotometrically. The degradation of naphthalene was determined quantitatively by High Performance Liquid Chromatography (HPLC) and qualitatively by GC/MS to determine the Intermediates resulted from degradation. The five most promising naphthalene degrading bacterial isolates were grown on five concentrations of naphthalene. Four out of the five bacterial isolates reached their maximum growth at the 7th day. The most naphthalene degrading bacterial 29 isolates was MAM-P22, the degradation rate of naphthalene in concentration of 7 mM by Bordetella avium strain MAM-P22 was 95%. This isolated bacterial strain was shown to be able to utilize naphthalene as a sole carbon and energy source. Degradation rate of Naphthalene was evaluated by chromatographic analysis after 21 days of incubation as shown in Fig 6, this result showed that most efficient bacterial isolate was MAM-P22 at the low and high concentration, MAM-P22 degraded more than 93% at the four concentrations of Naphthalene The results also revealed that the degradation percentage of Naphthalene was ranged between 84% up to 93% of 7(mM) naphthalene, and ranged from 95% to 98% of 4–6 (mM) naphthalene. The degradation percentage of naphthalene was concentration dependant (i.e., as the concentration increased the degradation decreased). Fig 6: Degradation percentage of naphthalene after 21 days by HPLC (Abo-State et.al,2017) The MAM-P22 strain degraded 95% of the highest concentration 7 mM. The results of GC/MS analysis revealed that Bordetella avium MAM-P22 degraded Naphthalene to give six intermediate compounds, These compounds were 1,2-Benzene dicarboxylic acid, Butyl-2,4dimethyl-2-nitro-4-pentenoate, 1-Nonen-3-ol, Eicosane, Nonacosane, 30 Fig 7: Naphthalene degradation pathway by MAM-P22 strain (Abo-State et.al,2017) Bordetella avium MAM-P22 strain makes naphthalene undergo oxidation followed by ring fission to give 1,2-Benzene dicarboxylic acid, 4-methyl-dimethyl ester and more oxidation followed by ring fission to give Butyl-2,4-dimethyl-2-nitro-4-pentenoate and reduction followed demethylation to give 1-Nonen-3-ol and more reduction and polymerization 31 followed by methylation to give Eicosane and Nonacosane intermediate compounds formed which undergo a kind of polymerization of aliphatic compounds. In 2016, Abarian et.al at the University of Kernan, Iran conducted a study to isolate and identify bacteria that could degrade naphthalene, from three regions of the Gol Gohar Mine at Sirjan, Iran. Water samples were collected from the superficial layer (0–10cm) from three contaminated places in the Gol Gohar Mine. Total naphthalene degrading bacteria were quantified with the most probable number (MPN) procedure using microtiter plates and colony forming unit (CFU) method. All plates were incubated at 30±1°C. After seven days, the numbers of grown colonies were counted. Growth curves of the isolates were commonly estimated indirectly by turbidity estimation as (O.D. at 600 nm). The growth rate of selected bacterial strains in various concentrations of naphthalene was determined. The Bushnell Hass (BH) medium was supplemented with various concentrations of naphthalene (0.02%, 0.03%, 0.04%, 0.05% and 0.06%, and Gas Chromatography (GC) was used to precisely determine naphthalene degradation. Twenty-two naphthalene-degrading bacteria were isolated from enrichment mine water cultures that were incubated at 30ºC for seven days. The screening results showed that all isolated bacteria could degrade naphthalene at a concentration of 200 mg/l. Seven isolates were selected for capability of growth on a higher concentration of naphthalene (300- 400 mg/l). From these isolates, strains 79N and 72N were able to utilize high concentrations of naphthalene (600 mg/l), therefore as a next step, phylogenetic molecular determination of the isolates was carried out by amplification and sequencing the 16S rRNA gene and comparing the sequences to the database of known 16S rRNA sequences. The molecular determination showed that these two isolated strains (79N and 72N) belonged to Pseudomonas gessardii AHB79N and the strain 72N was related to Pseudomonas fluorescens AHB72N. The results of GC analysis of residual naphthalene at BH medium showed that the Pseudomonas gessardii AHB79N strain was the best naphthalene degrader and degraded 55% of naphthalene at 600 mg/l concentration after seven days. Coming to removal of toluene from wastewater one of the earlier studies by Mehdizadeh (Mehdizadeh S.N, et al 2011) showed they used a pure culture of Alcaligenese faecalis to treat cryogenically preserved bacteria were incubated with sterile medium containing casein peptone (10g/L), yeast extract (5 g/L) and NaCl (10g/L) in 250 mL Erlenmeyer flasks in order to increase cell density in an incubator under 25°C and at 150 rpm by shaking for 24 h. The final cell concentration OD600 of the preculture reached 1.28. Gas chromatography was 32 used to measure toluene and hexadecane concentrations. The possibility of biodegradation of toluene at high initial concentrations (up to 1 g/L) was investigated in the biodegradation process in EMBRs (Extractive membrane bioreactor system). The initial concentrations of toluene were varied from 50 to 1,000 mg/L. The temperature in the bioreactor was controlled at 25 C and the pH set at 7 and the stirrer speed was 200 rpm. The air flow rate was 2 L/min. In order to investigate the biodegradation of toluene, 500 mL of fresh synthetic wastewater with 400 mg/L toluene was prepared and circulated in the membrane tubing. In order to evaluate growth kinetics, the biomass growth data from different initial toluene degradation batch experiments were plotted on a semi-logarithmic graph. After short lag phase, linear plots were obtained at all initial concentrations, which indicated that the cultures were growing exponentially in this region. The specific growth rates (µ) were calculated from the slope of the logarithm of the biomass concentration vs. time curves in the linear range. Toluene has a toxic nature and an inhibitory effect on cell growth and the Andrews-Haldane growth kinetics equation can represent the growth kinetics on this substrate. Cell Growth Kinetics in a batch reactor during the exponential phase, is modelled by the following equation: 𝑑𝑋 𝑑𝑡 = 𝜇𝑋 ……………………………………….. (i) Haldane’s inhibitory growth kinetics is as follows: 𝜇= 𝜇𝑚𝑎𝑥 𝑆 𝑆2 ) 𝑘1 𝑘𝑠 +𝑆+( ……………………(ii) S is the liquid substrate concentration; 𝜇 the specific growth rate; X, the biomass concentration; 𝜇𝑚𝑎𝑥 the maximum specific growth rate; fej, 𝑘𝑠 the Monod half-saturation constant and 𝑘1 is the substrate inhibition constant. Term 𝑘𝑠 is that value of the limiting nutrient concentration at which the specific growth rate is half of its maximum value. In the time course profile of toluene degradation and cell growth in the EMBR, the dramatic decline of toluene concentration occurred in the initial hours due to its transfer into the bio media and consumption of toluene by the bacterial culture. After the growth phase of the bacterial population, the cell concentration increased and the toluene decreased slowly. This was because of deduction in dissolved oxygen and nitrogen source in the bio media. Over the concentration ranges tested, A. faecalis could degrade high toluene concentrations up to 1 g/L. 33 Fig 8: Time course profile of toluene biodegradation in an EMBR (Mehdizadeh S.N, et al 2011) It was concluded that this microorganism is a suitable choice for biological processes in which the risk of surges in pollutant load is high. The Haldane-Andrews model described the growth kinetics of A. faecalis on toluene as the substrate. Various new strains of petroleum-hydrocarbon degrading bacteria like Citrobacter , Klebsiella , Acinetobacter, Pseudomonas, Bacteroides ,Clostridium etc are constantly being studied to evaluate the efficacy to degrade toluene and other hydrocarbons. A toluenedegrading strain was isolated from active sludge of wastewater treatment plant and its growth characteristics was studied and was identified by 16s rDNA sequencing. (Men J., Cheng F. 2011). For culture enrichment, 10 g activated sludge was added into 100 ml sterile water to get a uniform suspended liquid. Then 10 ml of suspension was added into 90 ml of inorganic nutrient medium with 1% (V/V) liquid toluene as carbon source. The enriched consortium was transferred to the plate filled with toluene-added medium. After 2 days cultivation, the colonies on culture plate were selected and pure culture was added into the liquid medium. Then the cultivation procedure mentioned earlier was repeated twice. Finally, one microbial strain which could utilize toluene as the sole carbon source was acquired. To test potential degradation of the strain on toluene, both the toluene concentration profile and microbial growth characteristics were examined. For the identification of the bacteria, 16s rDNA sequencing was conducted using PCR and after its purification the nucleotide sequence was compared with GenBank using BLAST tool. The experimental data of toluene degradation were further used for kinetic analysis. The relationship between the toluene removal ln(c0/c) and reaction time (t) (c0= 1.19 and 2.45 34 mg/l) suggested that the biodegradation reaction of toluene followed first-order kinetics, defined as the following equations : - 𝑑𝑐 𝑑𝑡 = 𝑘𝑐 ………. (iii) ln(c0/c) = kt ……….(iv) where, toluene removal rate constant is (k), the average removal rate of toluene is (dc/dt) and c is the initial concentrations. Fig 9: Relationship between toluene removal ln(c0/c) and reaction time t. By evaluating the toluene removal efficiency, the results showed that the strain was able to grow on toluene as its sole carbon source. After 24 h, the residual toluene concentrations in the all tested initial concentrations declined below 0.5 mg/l . The removal efficiency of 94.1% of toluene was observed in the low initial concentration of 1.19 mg/l. The removal efficiencies were almost the same with the increase of initial concentrations. Values of removal efficiencies were 93.3 and 92.5% when the initial concentrations were raised to 3.28 and 6.17 mg/l, respectively. The OD600 values after 24 h cultivation of the isolated strain under a wild temperature and pH range was analysed which gave the optimum temperature and pH as 30°C and 6.5 pH respectively. The isolated strain in this study, designated MJ001, was an aerobic Gram-negative motile rod, with size of 0.5 to 0.7μm in width and 1.5 to 2.5μm in length and having a flagellum. Taxonomical identification revealed 100% similarity to Pseudomonas sp. In this study, this strain was designated as Pseudomonas sp. strain MJ001. 35 Fig 10: Toluene concentration profile during degradation process under various initial toluene concentrations (other conditions as following: initial OD600= 0.05, pH= 6.5, temperature was maintained at 30°C). Thus, new strains and their potentials in degrading toluene and other hydrocarbons are continuously studied for enhanced bioremediation. In another approach, to clean up petroleum-hydrocarbon polluted groundwater, sulphate reduction mechanisms were applied to check the subsequent toluene and other hydrocarbon degradation (Huang W.-H. et al 2017). Sulphate-reducing bacteria (SRB) use organic compound (e.g., petroleum hydrocarbon) as the electron donor and sulphate as the electron acceptor. Sulphate reduction process will produce sulphide, which can react with cations (e.g., metals ions) and form insoluble precipitates thus causing bioprecipitation. SRB can oxidize petroleum hydrocarbons and transform the sulphate to sulphide via sulphate-reducing reactions. The stoichiometric equation for the sulphate reduction of petroleum hydrocarbons (using toluene as the target compound) is as follows: C7H8 + 4.5 SO42- + 9 H+ -→ 2.25 H2S + 2.25 HS- + 7 CO2 + 4 H2O Hydrogen sulphide will then react with metal ions and produce precipitates of metal sulphide by the following equation: M + H2S -→ MS(S) +2H+ Groundwater at an industrial site located inside an industrial park in southern Taiwan was contaminated by toluene and copper. In this research, a batch microcosm study was conducted to evaluate the mechanisms and effectiveness of simultaneous removal of toluene 36 and copper from contaminated groundwater via the anaerobic sulphate-reducing reactions. The anaerobic microcosm was constructed with 35 mL of contaminated groundwater (containing toluene and copper), 20 g of site soils, and 5 mL of sludge (inoculate) (or 5 mL of mineral medium in control microcosms) in a glass serum bottle (70 mL). Sodium sulphate was added into the sulphate reduction and kill control microcosms (Groups SR and KC) for sulphate supplement, and the initial sulphate concentration was 236 mg/L in microcosms. The initial toluene and copper concentrations in all microcosms were 17.5 and 12 mg/L, respectively. Sulphate in site groundwater ranged from 12 to 23 mg/L. The changes in toluene and copper concentrations during the 40-day operational period was evaluated. Approximately 99% of toluene and copper could be removed during the 40-day operational period. In SR microcosms, toluene concentrations dropped from 17.5 to 5 and 0.1 mg/L after 10 and 26 days of operation, respectively. In LC microcosms, toluene concentrations dropped from 17 to 8 and 3 mg/L after 10 and 26 days of incubation, respectively. Results show that toluene concentrations dropped significantly in both LC (live control group) and SR (sulphate reduction group) microcosms, and higher toluene removal rate was observed in SR microcosms. Results demonstrated that the concentrations of copper in SR microcosms dropped from 12.3 to 4.5 and 0.1 mg/L after 10 and 37 days of incubation, respectively. However, only slight decrease in copper concentration was detected in LC bottles during the operational period (decreased from 11.8 to 9.1 mg/L). Results suggest that the occurrence of bioprecipitation mechanism could be the cause of copper removal during the sulphate reducing process. Without sulphate supplement, significant copper removal was not observed. Fig 11: Changes in toluene concentrations during the 40-day operational period. 37 DGGE technique was applied to evaluate changes of predominant bacteria, which were responsible for the toluene biodegradation and copper bioprecipitation. To identify representative bacteria for contaminant removal, DGGE bands were eluted, amplified, and sequenced for the 16S rDNA. The identities of the nucleotide sequences of 12 dominant bacteria ranged were in the range from 97 to 100% of specific microorganisms comparing to GenBank database. The major findings from this study included the following: (1) simultaneous toluene and Cu removal was achieved under sulphate reduction, (2) toluene removal was enhanced with sulphate addition via anaerobic conditions, (3) Cu was removed via bioprecipitation mechanisms, and (4) produced sulphide reacted with Cu and formed cooper sulphide via sulphate reduction. Results would be useful in designing a practical system for in situ treatment of polluted sites contaminated with petroleum hydrocarbons and heavy metals. 38 Acknowledgement The satiation and euphoria that accompany the successful completion of this project would be curtailed without the mention of the people who made it possible. It is a genuine pleasure of mine to express my hearty gratitude to Dr. Bhaswati Chakraborty, Assistant Professor, Department of Biotechnology, for giving me an opportunity to work on this project. Her constant support, guidance and encouragement always inspires me to do better. I owe a deep sense of gratitude to her for her priceless comments and feedback. She has been available for support throughout the period of my project. Any omission in this brief acknowledgement does not mean lack of gratitude. 39 10. Bibliography 1) Readman JW, Fillmann G, Tolosa I, Bartocci J, Villeneuve JP, Catinni C, et al. (2002) Contamination of the Black Sea. Marine Pollution Bulletin. 2002; 44:48-62. DOI: 10.1016 2) Aggarwal VR, McBeth J, Zakrzewska JM, Lunt M, Macfarlane GJ (2006) The epidemiology of chronic syndromes that are frequently unexplained: do they have a common associated factor? Int J Epidemiol 35(2): 468-476 3) Das N, Chandran P. (2011), Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnology Research International. 2011:1-13. DOI: 10.4061/2011/941810 4) S. N. Mehdizadeh, M. R. Mehrnia, K. Abdi and M. H. Sarrafzadeh,(2011) Biological treatment of toluene contaminated wastewater by Alcaligenese faecalis in an extractive membrane bioreactor; experiments and modelling, Water Science & Technology , 2011, 64(6), Pages 1239-1245 5) Men J., Cheng F.2011,Biodegradation and growth characteristics of a toluene degrading strain, African Journal of Biotechnology, 2011, Vol. 10(61), pp. 1329913306. 6) Abha S, Singh CS. (2012) Hydrocarbon pollution: Effects on living organisms, remediation of contaminated environments and effects of heavy metals cocontamination on bioremediation 7) OB Akpor, UF Akolmiko, TD Olau and BI Aderiye (2014). Remediation of polluted wastewater effluents: Hydrocarbons removal. Biochemistry unit, Department of Biological Sciences, Kwara state, Nigeria. 8) Chen, M.; Xu, P. Zeng, G.; Yang, C.; Huang, D.; Zhang, J. (2015), Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting. applications, microbes and future research needs. Biotechnology. Adv, 33, 745–755 9) Guillermo Ladino-Orjuela, Roberto Da Silva, Eleni Gomes, John Parsons (2015), Metabolic pathways for degradation of aromatic hydrocarbon by bacteria, Reviews of Environmental Contamination and Toxicology 40 10) Behrooz Karimi, Maryam Habibi and Mehry Esvand (2015), Biodegradation of naphthalene using Pseudomonas aeruginosa by up flow anoxic–aerobic continuous flow combined bioreactor, Journal of environmental health, science and engineering 11) Moslem Abarian, Mehdi Hassanshahian, Arastoo Badoei-Dalfard (2016) Degradation of naphthalene by bacterial isolates from the Gol Gohar Mine, Iran, Department of Biology, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran 12) Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. (2016) Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiology. 745-769 13) Moslem Abarian, Mehdi Hassanshahian1,Arastoo Badoei-Dalfard (2016), Degradation of naphthalene by bacterial isolates from the Gol Gohar Mine, Iran, Progress in Biological Sciences 171-180 14) Abiodun O. Adeniji, Omobola Okoh and Anthony. (2017). Petroleum Hydrocarbon Profiles of water and sediment of Algoa Bay, Eastern Cape, South Africa. Journal of Hazardous Materials 15) M.A.M. Abo-State, B.Y. Riad, A.A. Bakr, M.F. Abdel Aziz (2017) Biodegradation of naphthalene by Bordetella avium isolated from petroleum refinery wastewater in Egypt and its pathway. Journal of Radiation Research and Applied Sciences 16) . Wei-Hsiang Huang, Cheng-Di Dong, Chiu-Wen Chen, Rao Y. Surampalli, Chi-Ming Kao,2017,Application of sulphate reduction mechanisms for the simultaneous bioremediation of toluene and copper contaminated groundwater, International Biodeterioration & Biodegradation, Volume 124, 2017, Pages 215-222, ISSN 09648305, 17) Yue-Hui Hong1, Cong-Cong Ye1, Qian-Zhi Zhou1, Xiao-Ying Wu2, Jian-Ping Yuan1, Juan Peng1, Hailin Deng1and Jiang-Hai Wang (2017). Genome Sequencing Reveals the Potential of Achromobacter sp. HZ01 for Bioremediation 18) Fowzia Ahmed and ANM Fakhruddin (2018). A Review on Environmental Contamination of Petroleum Hydrocarbons and its Biodegradation, Department of Environmental Sciences, Jahangirnagar University, Bangladesh 19) Reddy, P.V.; Karegoudar, T.B.; Nayak, A.S. (2018) Enhanced utilization of fluorene by Penicillium sp. PRNK-: Effect of rhamnolipid biosurfactant and synthetic surfactants. 2018, 151, 206–211 20) Roy, A.; Dutta, A.; Pal, S.; Gupta, A.; Sarkar, J.; Chatterjee, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K (2018) Bio stimulation and bioaugmentation of native microbial 41 community accelerated bioremediation of oil refinery sludge. Bioresources. Technology 2018, 253, 22–32 21) Cecotti, M.; Coppotelli, B.M.; Mora, V.C.; Viera, M.; Morelli, I.S. (2018) Efficiency of surfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarboncontaminated soil: Link with bioavailability and the dynamics of the bacterial community. Sci. Total Environ. 2018, 634, 224–234 22) Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. (2018) Bioavailability of weathered hydrocarbons in engine oil-contaminated soil: Impact of bioaugmentation mediated by Pseudomonas spp. on bioremediation. Sci. Total Environ. 2018, 636, 968–974. 23) Safdari, M.-S.; Kariminia, H.-R.; Rahmati, M.; Fazlollahi, F.; Polasko, A.; Mahendra, S.; Wilding, W.V.; Fletcher, T.H. (2018) Development of bioreactors for comparative study of natural attenuation, bio stimulation, and bioaugmentation of petroleumhydrocarbon contaminated soil. J. Hazard. Mater. 2018, 342, 270–278. 24) Cecotti, M.; Coppotelli, B.M.; Mora, V.C.; Viera, M.; Morelli, I.S. (2018) Efficiency of surfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarboncontaminated soil: Link with bioavailability and the dynamics of the bacterial community. Sci. Total Environ. 2018, 634, 224–234 25) Manish Srivastava, Anamika Srivastava, Anjali Yadav and Varun Rawat (2019). Source and Control of Hydrocarbon Pollution. 26) Koolivand, A.; Abtahi, H.; Parhamfar, M.; Didehdar, M.; Saeedi, R.; Fahimirad, S. (2019) Biodegradation of high concentrations of petroleum compounds by using indigenous bacteria isolated from petroleum hydrocarbons-rich sludge: Effective scale-up from liquid medium to composting process. J. Environ. Manag.2019, 248, 109228 27) Wo´zniak-Karczewska, M.; Lisiecki, P.; Białas, W.; Owsianiak, M.; PiotrowskaCyplik, A.; Wolko, Ł.; Ławniczak, Ł.; Heipieper, H.J.; Gutierrez, T.; Chrzanowski, Ł. (2019). Effect of bioaugmentation on long-term biodegradation of diesel/biodiesel blends in soil microcosms. Sci. Total Environ. 2019, 671, 948–958. 28) Chaudhary D., Dong-Uk Kim, Dockyu Kim & Jaisoo Kim (2019), Flavobacterium petrolei sp. nov., a novel psychrophilic, diesel-degrading bacterium isolated from oilcontaminated Arctic soil 42 29) Oualha, M.; Al-Kaabi, N.; Al-Ghouti, M.; Zouari, N. (2019) Identification and overcome of limitations of weathered oil hydrocarbons bioremediation by an adapted Bacillus sorensis strain. J. Environment Management. 2019, 250, 109455. 30) Lu, L.; Zhang, J.; Peng, C (2019) Shift of Soil Polycyclic Aromatic Hydrocarbons (PAHs) dissipation pattern and microbial community composition due to rhamnolipid supplementation. Water Air Soil Pollution. 2019, 230, 107. 31) Czarny, J.; Staninska-Pi˛eta, J.; Piotrowska-Cyplik, A.; Wolko, Ł.; Staninski, K.; Hornik, B.; Cyplik, P (2019). Assessment of soil potential to natural attenuation and autochthonous bioaugmentation using microarray and functional predictions from metagenome profiling. Ann. Microbiology. 2019, 69, 945–955. 32) Leili Mohammad, Abbas Rahdar, Edris Bazrafshan, Hamid Dahmardeh 4, Md. Abu Bin Hasan Susan and George Z. Kyzas (2020). Petroleum Hydrocarbon Removal from Wastewaters: A Review 33) Łukasz Ławniczak, MartaWo´zniak-Karczewska, Andreas P. Loibner, Hermann J. Heipieper and Łukasz Chrzanowski (2020). Microbial Degradation of Hydrocarbons— Basic Principles for Bioremediation: A Review 34) Chiara Daccò, Lidia Nicola, Marta Elisabetta Eleonora Temporiti, Barbara Mannucci, Federica Corana, Giovanna Carpani and Solveig Tosi (2020), Trichoderma: Evaluation of Its Degrading Abilities for the Bioremediation of Hydrocarbon Complex Mixtures 35) Munazzam Jawad Shahid, Ameena A. AL-surhanee, Fayza Kouadri. Shafaqat Ali Neeha Nawaz, Muhammad Afzal, Muhammad Rizwan, Basharat Ali and Mona H. Soliman (2020), Review, Role of microorganisms in the remediation of wastewater in floating treatment wetlands: A Review,724–751.